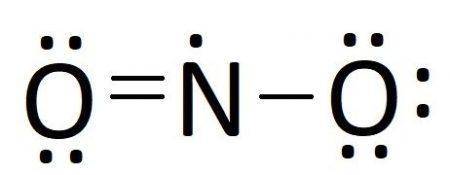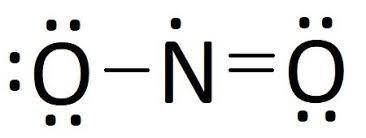Physics, 27.02.2020 03:16 amiechap12
Calculate the formal charge on the indicated atom in each of the following molecules or ions. Some molecules may have more than one possible resonance structure, each resulting in a different possible formal charge on the indicated atom. In such cases, supply both unique formal charge values separated by commas. Part Cnitrogen in NO2If there is more than one answer, separate them by commas.

Answers: 1
Another question on Physics

Physics, 21.06.2019 15:00
Does this organism still meet the definition of a eukaryote ? why or why not?
Answers: 2

Physics, 22.06.2019 11:20
If the radius of curvature of the cornea is 0.75 cm when the eye is focusing on an object 36.0 cm from the cornea vertex and the indexes of refraction are as described before, what is the distance from the cornea vertex to the retina? express your answer to two significant
Answers: 3

Physics, 22.06.2019 14:20
What are the starting materials for nuclear fission? two small nuclei two large nuclei a neutron and a large nucleus a neutron and a small nucleus
Answers: 2

Physics, 22.06.2019 18:30
In which situation is the acceleration of a car negative? question 5 options: a)the velocity of a car was 75 km/hr over 4 hours. b)the velocity of a car reduced from 50 km/hr over one minute. c)the velocity of a car increased from 40 km.h to 75 km/h over 15 minutes. d)the velocity of a car was 45 km/hr at 2: 00pm and at 4: 00pm its velocity was 85 km/ht.
Answers: 2
You know the right answer?
Calculate the formal charge on the indicated atom in each of the following molecules or ions. Some m...
Questions


English, 25.11.2020 22:40

Mathematics, 25.11.2020 22:40

Mathematics, 25.11.2020 22:40



Mathematics, 25.11.2020 22:40



Mathematics, 25.11.2020 22:40

Mathematics, 25.11.2020 22:40



Mathematics, 25.11.2020 22:40

Mathematics, 25.11.2020 22:40

Mathematics, 25.11.2020 22:40

World Languages, 25.11.2020 22:40

History, 25.11.2020 22:40

English, 25.11.2020 22:40

Mathematics, 25.11.2020 22:40