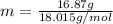Physics, 04.03.2020 23:32 jhenifelix
How much energy does it take to melt a 16.87 g ice cube? ΔHfus = 6.02 kJ/mol How much energy does it take to melt a 16.87 g ice cube? = 6.02 kJ/mol 108 kJ 102 kJ 5.64 kJ 936 J none of the above

Answers: 3
Another question on Physics

Physics, 22.06.2019 01:30
Use the frequency histogram to complete the following parts. ​(a) identify the class with the​ greatest, and the class with the​ least, relative frequency. ​(b) estimate the greatest and least relative frequencies. ​(c) describe any patterns with the data. female fibula lengths 30.5 31.5 32.5 33.5 34.5 35.5 36.5 37.5 38.5 39.5 0 0.05 0.1 0.15 0.2 0.25 length (in centimeters) relative frequency a histogram titled "female fibula lengths" has a horizontal axis labeled "length in centimeters" from 30.5 to 39.5 in increments of 1 and a vertical axis labeled "relative frequency" from 0 to 0.25 in increments of 0.05. the histogram contains vertical bars of width 1, where one vertical bar is centered over each of the horizontal axis tick marks. the approximate heights of the vertical bars are listed as follows, where the label is listed first and the approximate height is listed second: 30.5, 0.02; 31.5, 0.04; 32.5, 0.05; 33.5, 0.13; 34.5, 0.22; 35.5, 0.25; 36.5, 0.13; 37.5, 0.06; 38.5, 0.09; 39.5, 0.01. ​(a) the class with the greatest relative frequency is nothing to nothing centimeters. ​(type integers or decimals. do not round. use ascending​ order.)
Answers: 3


Physics, 22.06.2019 06:00
Using a pedometer, you walk 3000 steps in 20 minutes, so your speed is 150 steps/min. each of your steps is 0.7 m long. what is your speed? 150 steps/min=/s=/h
Answers: 1

Physics, 22.06.2019 09:00
In the first law of thermodynamics, triangle e=q-w, what does q stand for
Answers: 1
You know the right answer?
How much energy does it take to melt a 16.87 g ice cube? ΔHfus = 6.02 kJ/mol How much energy does it...
Questions

Social Studies, 31.01.2020 08:55



Social Studies, 31.01.2020 08:55

History, 31.01.2020 08:55

Mathematics, 31.01.2020 08:55




History, 31.01.2020 08:56

Mathematics, 31.01.2020 08:56


History, 31.01.2020 08:56



Advanced Placement (AP), 31.01.2020 08:56


Computers and Technology, 31.01.2020 08:56


English, 31.01.2020 08:56

 x m ...............................................1
x m ...............................................1