
Two containers hold an ideal gas at the same temperature and pressure. Both containers hold the same type of gas, but container B has twice the number of particles and twice the volume of container A. The average kinetic energy per molecule in container B is :
A. Twice that for container A
B. The same as that for container A
C. Half that for container A
D. Impossible to determine

Answers: 2
Another question on Physics

Physics, 22.06.2019 09:00
In the first law of thermodynamics, triangle e=q-w, what does q stand for
Answers: 1

Physics, 22.06.2019 12:50
The vapour pressure of benzene is 53.3 kpa at 60.6 °c, but it fell to 51.5 kpa when 19.0 g of a non-volatile organic compound was dissolved in 500 g of benzene. calculate the molar mass of the compound.
Answers: 2


Physics, 22.06.2019 16:30
Humidity is to blame for that muggy, steamy feeling you experience on some hot summer days. what gas in the atmosphere causes humidity? a) oxygen b) hydrogen c) nitrogen d) water vapor
Answers: 1
You know the right answer?
Two containers hold an ideal gas at the same temperature and pressure. Both containers hold the same...
Questions


History, 16.09.2019 01:50



Chemistry, 16.09.2019 01:50

Biology, 16.09.2019 01:50

History, 16.09.2019 01:50



Chemistry, 16.09.2019 01:50


Physics, 16.09.2019 01:50







Mathematics, 16.09.2019 01:50

Physics, 16.09.2019 01:50

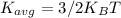
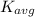 is the average kinetic energy per molecule and
is the average kinetic energy per molecule and 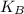 is the Boltzmann constant
is the Boltzmann constant 

