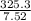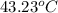Physics, 07.03.2020 05:05 ceeejay0621
A stream of warm water is produced in a steady-flow mixing process by combining 1.0 kg/s of cool water at 25 °C with 0.8 kg/s of hot water at 75 °C. During mixing, heat is lost to the surroundings at the rate of 30 kJ/s. What is the temperature of the warm water stream? Assume the specific heat of water is constant at 4.18 kJ/(kg·K).

Answers: 2
Another question on Physics

Physics, 22.06.2019 03:00
Isla’s change in velocity is 30 m/s, and hazel has the same change in velocity. which best explains why they would have different accelerations? isla had negative acceleration, and hazel had positive. isla had a different time than hazel. isla had positive acceleration, and hazel had negative. isla went a farther distance than hazel.
Answers: 1

Physics, 22.06.2019 12:00
If two students are running down the hall toward each other, trying to get to class, and they have the same mass and acceleration, what will happen when they collide? will their forces cancel out or will each one experience a reaction?
Answers: 1

Physics, 22.06.2019 14:40
On a geologic map, if the contacts between sedimentary rock units form a bull’s-eye pattern of concentric circles, with the youngest unit in the center, the underlying structure is a(n)
Answers: 3

Physics, 22.06.2019 21:10
Two masses are connected by a rope that hangs over a pulley as shown below. the pulley is a uniform cylinder of radius 푅=0.381meters and mass 푀=3.1푘푔. initially 푚"is on the ground and 푚#rests 2.5 meters above the ground. if the system is released, use conservation of energy to determine the speed of 푚#just before it strikes the ground. assume the pulley bearing is frictionless.
Answers: 2
You know the right answer?
A stream of warm water is produced in a steady-flow mixing process by combining 1.0 kg/s of cool wat...
Questions



Social Studies, 17.04.2020 21:36



Social Studies, 17.04.2020 21:36

Social Studies, 17.04.2020 21:36


Advanced Placement (AP), 17.04.2020 21:36



Mathematics, 17.04.2020 21:36


Mathematics, 17.04.2020 21:36

English, 17.04.2020 21:36





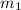 = 0.8 kg/s = 800 g,
= 0.8 kg/s = 800 g, 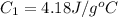
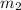 = 1 kg = 1000 g,
= 1 kg = 1000 g, 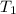 = (75 - T),
= (75 - T),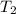 = T - 25
= T - 25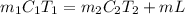
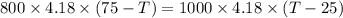 + 30000
+ 30000