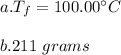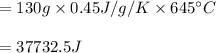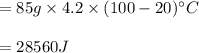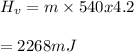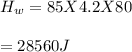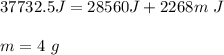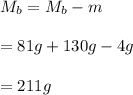You cool a 130.0 g slug of red-hot iron (temperature 745 ∘C) by dropping it into an insulated cup of negligible mass containing 85.0 g of water at 20.0 ∘C. Assume no heat exchange with the surroundings. How do you do this?
Part A What is the final temperature of the water?
Part B What is the final mass of the iron and the remaining water?

Answers: 1
Another question on Physics

Physics, 21.06.2019 18:40
Carbon-14 is a radioactive element that undergoes beta decay. which force is responsible for allowing carbon-14 to become stable? electromagnetic gravitational weak nuclear strong nuclear
Answers: 3

Physics, 22.06.2019 05:00
Aperson standing in a canoe exerts a force of 700 n to throw an anchor over the side. find the acceleration of the canoe if the total mass of the canoe and the person is 100 kg?
Answers: 1


Physics, 22.06.2019 14:30
Alandscaper is shopping for landscaping materials. she wants to use materials through which water flows easily. which materials should she choose? check all that apply. clay gravel granite rocks with cracks loosely packed soil
Answers: 2
You know the right answer?
You cool a 130.0 g slug of red-hot iron (temperature 745 ∘C) by dropping it into an insulated cup of...
Questions


Spanish, 10.11.2020 04:50

Mathematics, 10.11.2020 04:50

Mathematics, 10.11.2020 04:50




Mathematics, 10.11.2020 04:50



Mathematics, 10.11.2020 04:50

English, 10.11.2020 04:50



Arts, 10.11.2020 04:50



Biology, 10.11.2020 04:50

Mathematics, 10.11.2020 04:50

Geography, 10.11.2020 04:50