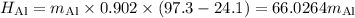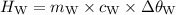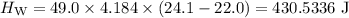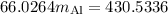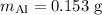A piece of aluminum metal at an initial temperature of 97.3°C was placed in a calorimeter containing 49.0g of water at an initial temperature of 22.0°C. The two were allowed to come to thermal equilibrium and the final temperature was 24.1°C. The specific heats of water and aluminum are 4.184 J/g °C and 0.902 J/g °C, respectively. What was the mass of the Al piece that was added?

Answers: 3
Another question on Physics

Physics, 21.06.2019 19:10
What happens to light waves from a star as the star moves away from earth?
Answers: 3

Physics, 22.06.2019 09:00
What is a possible result of higher air temperature caused by global warming
Answers: 1

Physics, 23.06.2019 00:00
You are standing on a boat. which of the following strategies will make the boat start moving? a. pushing the mast b. throwing some cargo out of the boat c. pushing the front ofthe boat d. pushing another passenger
Answers: 3

Physics, 23.06.2019 04:10
The release of energy by the nucleus of atom as a result of nuclear fission is radiant energy true false
Answers: 2
You know the right answer?
A piece of aluminum metal at an initial temperature of 97.3°C was placed in a calorimeter containing...
Questions

Biology, 07.12.2020 23:50


Health, 07.12.2020 23:50

Mathematics, 07.12.2020 23:50


Geography, 07.12.2020 23:50

Mathematics, 07.12.2020 23:50

Health, 07.12.2020 23:50

Arts, 07.12.2020 23:50

Mathematics, 07.12.2020 23:50


Social Studies, 07.12.2020 23:50



History, 07.12.2020 23:50

Mathematics, 07.12.2020 23:50




Arts, 07.12.2020 23:50