
Physics, 10.03.2020 03:07 lydiadmanautou04
Molybdenum (Mo) has a BCC crystal structure, an atomic radius of 0.1363 nm, and an atomic weight of 95.94 g/mol. Compute and compare its theoretical density with the experimental value found inside the front cover of the book.

Answers: 1
Another question on Physics

Physics, 22.06.2019 04:30
The pressure increases by 1.0 x 104 n/m^2 for every meter of depth beneath the surface of the ocean. at what depth does the volume of a pyrex (bulk modulus 2.6 x 1010n/m^2) glass cube, 9.8 x 10^−2m on an edge at the ocean's surface, decrease by 7.5 x 10−10m^3? explain the formula beyond this point: p=1.0x10^4, b=2.6x10^10, l=9.8x10^−2, delta v=7.5x10^−10. at some point l needs to be cubed. why p is divided by delta v?
Answers: 2

Physics, 22.06.2019 06:30
If an atom contains 11 protons in its nucleus, predict the element with the atomic number and electronic configuration. will the number of electrons remain the same during the chemical reaction in such an atom? explain.
Answers: 3

Physics, 22.06.2019 08:00
Choose each of the settings that follow and list them below do it ill mark brainliest first object= position= speed= acceleration= second object= position= speed= acceleration=
Answers: 2

Physics, 22.06.2019 09:00
Abicycle slows down when the rider applies the brakes. what type of energy transformation is involved in this example? a. kinetic energy into heat energy b. heat energy into potential energy c. potential energy into kinetic energy d. kinetic energy into mechanical energy
Answers: 1
You know the right answer?
Molybdenum (Mo) has a BCC crystal structure, an atomic radius of 0.1363 nm, and an atomic weight of...
Questions

Spanish, 14.12.2021 16:30

Physics, 14.12.2021 16:30



Mathematics, 14.12.2021 16:30

Mathematics, 14.12.2021 16:30



History, 14.12.2021 16:30






Mathematics, 14.12.2021 16:40

Mathematics, 14.12.2021 16:40




Mathematics, 14.12.2021 16:40

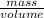
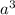 =
=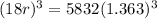 = 14, 767.434
= 14, 767.434 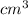
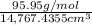 ×
× 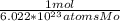
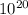 = 10.79 E18
= 10.79 E18

