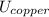Physics, 10.03.2020 04:37 eymurezgi1
A 50-kg iron block and a 20-kg copper block, both initially at 80°C, are dropped into a large lake at 15°C. Thermal equilibrium is established after a while as a result of heat transfer between the blocks and the lake water. Assuming the surroundings to be at 20°C, determine the amount of work that could have been produced if the entire process were executed in a reversible manner.

Answers: 1
Another question on Physics

Physics, 22.06.2019 05:00
The image below shows numerous volcanic mountains in the pacific northwest. what is the most likely cause of the volcanic and earthquake activity in this region?
Answers: 2


Physics, 22.06.2019 09:30
On a day when the barometer reads 75.23 cm, a reaction vessel holds 250 ml of ideal gas at 20 celsius. an oil manometer ( ρ= 810 kg/m^3) reads the pressure in the vessel to be 41 cm of oil and below atmospheric pressure. what volume will the gas occupy under s.t.p.?
Answers: 2

Physics, 22.06.2019 11:00
I'm thinking it's 2 you are asked to explain the earth's magnetic field. which is the best reply? 1. the earth's magnetic south is similar to the north pole of a magnet. 2. the earth's core has a strong magnetic charge similar to the south pole of a magnet. 3. the earth's geomagnetic south is similar to the south pole of a magnet. 4. the earth's magnetic charge is not centered at either pole; it varies based on location.
Answers: 1
You know the right answer?
A 50-kg iron block and a 20-kg copper block, both initially at 80°C, are dropped into a large lake a...
Questions




Physics, 22.01.2021 02:50


Spanish, 22.01.2021 02:50


History, 22.01.2021 02:50

Business, 22.01.2021 02:50

Chemistry, 22.01.2021 02:50


World Languages, 22.01.2021 02:50

English, 22.01.2021 02:50

Arts, 22.01.2021 02:50






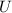 = Δ
= Δ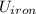 + Δ
+ Δ