
Physics, 10.03.2020 07:56 leonardoocampo7555
A jeweler needs to electroplate gold onto a bracelet using an ionic solution. He knows that the charge carriers in the ionic solution are gold ions of charge e, and that each gold ion has a mass of 22g. The gold ions move through the solution, and are deposited on the bracelet. He has calculated that he must deposit 0.20 g of 901d to reach the necessary thickness. If each gold ion has a mass of g, what current should he use to electroplate the bracelet in three hours

Answers: 3
Another question on Physics

Physics, 22.06.2019 11:30
Water is siphoned from a large tank and discharges into the atmosphere through a 50-mm diameter tube. the end of the tube is b = 2.1 m below the tank bottom which is a = 7.4 m deep, and viscous effects are negligible. determine the maximum height h over which the water can be siphoned without cavitation occurring. atmospheric pressure is 101.4 kpa, and the water vapor pressure is 1.79 kpa (absolute)
Answers: 3

Physics, 22.06.2019 15:30
The radius of a sphere is increasing at a rate of 9 cm/ sec. find the radius of the sphere when the volume and the radius of the sphere are increasing at the same numerical rate.
Answers: 1


Physics, 23.06.2019 00:00
What is the difference between mass density and weight density?
Answers: 1
You know the right answer?
A jeweler needs to electroplate gold onto a bracelet using an ionic solution. He knows that the char...
Questions


Mathematics, 16.07.2021 03:20








Mathematics, 16.07.2021 03:20


Health, 16.07.2021 03:20








Biology, 16.07.2021 03:20

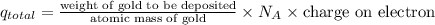
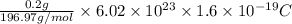
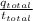
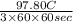
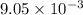 A
A

