
Temperature plays in important part in most chemical reactions. Generally speaking, the higher the temperature, the higher the rate of reaction due to the increased amount of energy available as well as an increase in particle contact.
Suppose a chemical reaction was discovered in which temperature was not relevant at all. An experiment was devised where all other factors were allowed to vary - pressure, number of particles, etc. - and the temperature was increased from 10°C to 50°C while the rate of the reaction was measured.
How would the graph seen here change with respect to this new reaction?
A) The graph would show a random scatter of data points.
B) The graph would be a single vertical line at some temperature.
C) The graph would be a single horizontal line at some reaction rate
D) The graph would not be significantly different from the one shown.
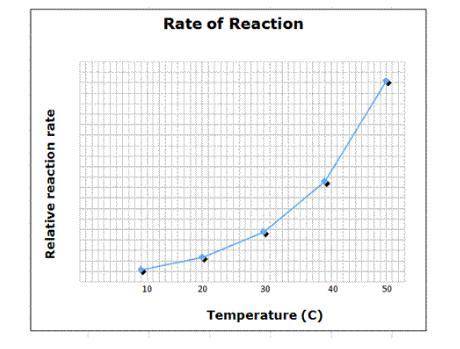

Answers: 3
Another question on Physics

Physics, 22.06.2019 03:00
1. a net force of 100 newton’s is applied to a wagon for 5 seconds. this causes the wagon to undergo a change in momentum of
Answers: 1


Physics, 22.06.2019 14:00
Explain why you think this diagram shows what happened to the carbon in the biodome.
Answers: 2

You know the right answer?
Temperature plays in important part in most chemical reactions. Generally speaking, the higher the t...
Questions


Mathematics, 06.05.2020 00:16

Biology, 06.05.2020 00:16

Social Studies, 06.05.2020 00:16




Advanced Placement (AP), 06.05.2020 00:16


Mathematics, 06.05.2020 00:16




Biology, 06.05.2020 00:16








