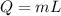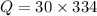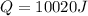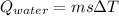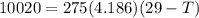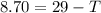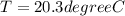Physics, 14.03.2020 06:12 karizm2010
An ice cube with mass mi=30 g and temperature T=0℃ completely melts in a cup of water. The mass of the water in the cup is mw=275 g and its initial temperature, before dropping the ice cube in it, is Tw=29℃. Calculate the temperature of the water in the cup at the instant the ice cube has completely melted. The specific heat capacity of water is cw=4.186 J/(g℃) and the latent heat of fusion of ice is Lf=334 J/g.

Answers: 3
Another question on Physics

Physics, 22.06.2019 03:00
The passage that directs air to the lungs is called a. diaphragm. b. larynx. c. bronchi. d. pharynx.
Answers: 2

Physics, 22.06.2019 18:10
Which of the following statement is false ? a.batteries should never be stored on metal trays or outside in the elements)b, battery acid cannot be neutralized)c.do not stack batteries on top of each other)d.never put a damaged battery in a dumpster. i
Answers: 1

Physics, 23.06.2019 07:00
Which best offense of relationship between speed and velocity speed is velocity what displacement velocity and speed with displacement spee is based on a specific direction velocity isis based on displacement
Answers: 1

Physics, 23.06.2019 08:30
The lift raises a car of mass 850 kg to a height of 2.1 m. it uses a force of 8330 n and takes 4 seconds to raise the car. what is the power of the lift?
Answers: 1
You know the right answer?
An ice cube with mass mi=30 g and temperature T=0℃ completely melts in a cup of water. The mass of t...
Questions

Health, 13.10.2020 05:01

Mathematics, 13.10.2020 05:01

Mathematics, 13.10.2020 05:01

Mathematics, 13.10.2020 05:01




Geography, 13.10.2020 05:01

Mathematics, 13.10.2020 05:01








Mathematics, 13.10.2020 05:01

Chemistry, 13.10.2020 05:01