
Physics, 16.03.2020 19:16 msladycie8831
Which of the following are decomposition reactions?
A. 2LiOH (s) + CO² (g) --> Li²CO³ (s) + H²O (I)
B. K²CO³ (s) --> K²O (s) + CO² (g)
C. 2SO³ (g) --> 2SO² (g) + O² (g)
D. 2Na (s) + Cl² (g) --> 2NaCl (s)

Answers: 2
Another question on Physics

Physics, 22.06.2019 05:00
Which is the best predictor of the radioactive nature of an isotope? o the proton-to-electron ratio the neutron-to-proton ratio o the neutron-to-electron ratio the electron-to-proton ratio
Answers: 1

Physics, 22.06.2019 13:20
Ahanging spring stretches by 35.0 cm when an object of mass 450 g is hung on it at rest. in this situation, we define its position as x = 0. the object is pulled down an additional 18.0 cm and released from rest to oscillate without friction. what is its position x at a moment 84.4 s later? express your answer in cm.
Answers: 1

Physics, 22.06.2019 14:30
Suppose that 27 j of work is needed to stretch a spring from its natural length of 6 m to a length of 9 m. (a) how much work is needed to stretch the spring from 12 m to 14 m? j (b) how far beyond its natural length will a force of 78 n keep the spring stretched?
Answers: 2

Physics, 22.06.2019 23:00
Which type of reaction is shown in this energy diagram? energy products activation energy reactants time o a. endothermic, because energy is released to the surroundings o b. exothermic, because energy is absorbed from the surroundings o c. exothermic, because energy is released to the surroundings o d. endothermic, because energy is absorbed from the surroundings
Answers: 1
You know the right answer?
Which of the following are decomposition reactions?
A. 2LiOH (s) + CO² (g) --> Li²CO³ (s) +...
A. 2LiOH (s) + CO² (g) --> Li²CO³ (s) +...
Questions









Mathematics, 15.07.2019 20:30


Mathematics, 15.07.2019 20:30

Health, 15.07.2019 20:30




Mathematics, 15.07.2019 20:30


History, 15.07.2019 20:30


English, 15.07.2019 20:30

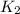
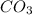 (s) -->
(s) --> 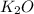 (s) +
(s) + 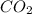 (g)
(g)
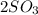 (g) -->
(g) --> 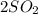 (g) +
(g) + 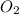 (g)
(g)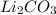 +
+ 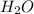
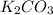 --->
---> 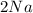 +
+ 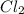 --->
---> 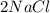
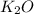 (s) +
(s) + 

