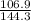You heat a 5.2 gram lead ball (specific heat capacity = 0.128 Joules/g-deg) to 183. ∘ C. You then drop the ball into 34.5 milliliters of water (density 1 g/ml; specific heat capacity = 4.184 J/g-deg ) at 22.4 ∘ C. What is the final temperature of the water when the lead and water reach thermal equilibrium?

Answers: 3
Another question on Physics

Physics, 21.06.2019 19:30
Identical marbles are released from the same height on each of the following four frictionless ramps . compare the speed of the marbles at the end of each ramp. explain your reasoning
Answers: 3

Physics, 22.06.2019 09:30
For this car, predict the shape of a graph that shows distance (x) versus time (t). note that time is the independent variable and will be on the bottom axis. need asap
Answers: 1

Physics, 22.06.2019 11:30
Which accounts for an increase in the temperature of a gas that is kept a constant volume
Answers: 1

Physics, 22.06.2019 12:50
The vapour pressure of benzene is 53.3 kpa at 60.6 °c, but it fell to 51.5 kpa when 19.0 g of a non-volatile organic compound was dissolved in 500 g of benzene. calculate the molar mass of the compound.
Answers: 2
You know the right answer?
You heat a 5.2 gram lead ball (specific heat capacity = 0.128 Joules/g-deg) to 183. ∘ C. You then dr...
Questions

Mathematics, 04.04.2021 03:30


Computers and Technology, 04.04.2021 03:30

Biology, 04.04.2021 03:40

Mathematics, 04.04.2021 03:40

Mathematics, 04.04.2021 03:40




Mathematics, 04.04.2021 03:40


Mathematics, 04.04.2021 03:40

Health, 04.04.2021 03:40


English, 04.04.2021 03:40




English, 04.04.2021 03:40

Mathematics, 04.04.2021 03:40

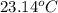
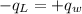 .............................1
.............................1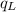 is the heat energy of the lead ball;
is the heat energy of the lead ball;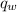 is the heat energy of water;
is the heat energy of water;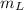 is the mass of the lead ball = 5.2 g
is the mass of the lead ball = 5.2 g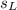 is the specific heat capacity of the lead ball = 0.128 Joules/g-deg
is the specific heat capacity of the lead ball = 0.128 Joules/g-deg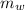 is the mass of water = volume x density = 34.5 ml x 1 g/ml = 34.5 g
is the mass of water = volume x density = 34.5 ml x 1 g/ml = 34.5 g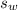 is the specific heat capacity of water = 4.184 J/g-deg
is the specific heat capacity of water = 4.184 J/g-deg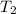 -22.4)
-22.4)