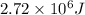Physics, 16.03.2020 22:38 fatherbamboo
Be sure to answer all parts. Isooctane (C8H18; d = 0.692 g/mL) is used as the fuel during a test of a new automobile engine. How much energy (in kJ) is released by complete combustion of 21.8 gal of isooctane to gases (ΔH o rxn = −5.44 × 103 kJ/mol)? Enter your answer in scientific notation.

Answers: 3
Another question on Physics

Physics, 21.06.2019 21:00
Apulley with a mechanical advantage of 5 will require you to pull times the amount of rope. a. 1/5 b. 5 c. 10 d. 15
Answers: 2

Physics, 22.06.2019 03:50
Aspecimen of oil having an initial volume of 580cm^3 is subjected to a pressure increase of 4.0 mpa, and the volume is found to decrease by 0.40 cm^3. (a) what is the bulk modulus of the material? (b) what is the compressibility of the material?
Answers: 1

Physics, 22.06.2019 14:30
Multiply or divide to make this english distance conversion. 174 inches = feet (14.5, 58, 2,088, 23)
Answers: 2

Physics, 22.06.2019 19:30
Water is siphoned from a large tank and discharges into the atmosphere through a 50-mm diameter tube. the end of the tube is b = 2.6 m below the tank bottom which is a = 6.7 m deep, and viscous effects are negligible. determine the maximum height h over which the water can be siphoned without cavitation occurring. atmospheric pressure is 101.4 kpa, and the water vapor pressure is 1.79 kpa (absolute). report your answer in meters to two decimal places.
Answers: 1
You know the right answer?
Be sure to answer all parts. Isooctane (C8H18; d = 0.692 g/mL) is used as the fuel during a test of...
Questions




English, 01.07.2021 01:00


Chemistry, 01.07.2021 01:00




Mathematics, 01.07.2021 01:00



Mathematics, 01.07.2021 01:00

English, 01.07.2021 01:00

Mathematics, 01.07.2021 01:00

English, 01.07.2021 01:00