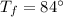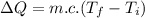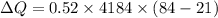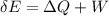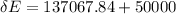Suppose you warm up 520 grams of water (about half a liter, or about a pint) on a stove, and while this is happening, you also stir the water with a beater, doing 5multiply. gif104 J of work on the water. After the large-scale motion of the water has dissipated away, the temperature of the water is observed to have risen from 21°C to 84°C.
What was the change in the thermal energy of the water?
deltacapEthermal =?
Taking the water as the system, how much energy transfer due to a temperature difference (microscopic work) Q was there across the system boundary?
Q = ?
Taking the water as the system, what was the energy change of the surroundings?
deltacapEsurroundings=?

Answers: 1
Another question on Physics

Physics, 21.06.2019 17:40
Which is not a source of noise pollution? a)airplanes b)acid rain c)machines d)cars
Answers: 2

Physics, 22.06.2019 02:40
If the wheels lock when braking suddenly the vehicle: a: loses traction b: lose steering wheel ability c: gain speed slightly d: gain steering ability
Answers: 1

Physics, 22.06.2019 14:20
What are the starting materials for nuclear fission? two small nuclei two large nuclei a neutron and a large nucleus a neutron and a small nucleus
Answers: 2

Physics, 22.06.2019 18:30
Anonzero net force acts on a particle and does work. which one of the following statements is true? the kinetic energy of the particle changes, but the speed of the particle does not change. the kinetic energy of the particle does not change, but the speed of the particle does change. the kinetic energy of the particle changes, but the velocity of the particle does not change. the kinetic energy and the speed of the particle change, but the velocity of the particle does not change. the kinetic energy, speed, and velocity of the particle change.
Answers: 1
You know the right answer?
Suppose you warm up 520 grams of water (about half a liter, or about a pint) on a stove, and while t...
Questions

Social Studies, 13.04.2020 21:19



History, 13.04.2020 21:20



Social Studies, 13.04.2020 21:20



History, 13.04.2020 21:20





English, 13.04.2020 21:20

Mathematics, 13.04.2020 21:20


Biology, 13.04.2020 21:20


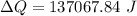 is the change in the thermal energy of water this is also the amount of energy crossing the system boundary due to temperature difference.
is the change in the thermal energy of water this is also the amount of energy crossing the system boundary due to temperature difference.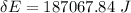
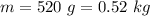 work done on the water by stirring,
work done on the water by stirring, 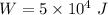 initial temperature of water,
initial temperature of water,  final temperature of water,
final temperature of water,