Physics, 17.03.2020 03:28 hannahgrac3
A 3.1-mole sample of an ideal gas is gently heated at constant temperature 290 K. The gas expands to 1.5 times its initial volume as its pressure decreases. What is the change in the internal energy of the gas? Let the ideal-gas constant R = 8.314 J/(mol • K).
3000 J
1500 J
0 J
-3000 J

Answers: 2
Another question on Physics

Physics, 21.06.2019 15:00
Answer asap! the weather patterns on earth are shown in this image. explain why there are convection currents; why there are hotter and colder parts of earth.
Answers: 1


Physics, 22.06.2019 06:00
An object moves from position +34m to the position -15m in 15 seconds. what is the total displacement? what is the total velocity?
Answers: 3

Physics, 22.06.2019 07:30
Write the function getkthdigit(n, k) that takes a possibly-negative int n and a non-negative int k, and returns the kth digit of n, starting from 0, counting from the right
Answers: 3
You know the right answer?
A 3.1-mole sample of an ideal gas is gently heated at constant temperature 290 K. The gas expands to...
Questions


Mathematics, 23.04.2021 22:20

Mathematics, 23.04.2021 22:20






Computers and Technology, 23.04.2021 22:20

Social Studies, 23.04.2021 22:20

Mathematics, 23.04.2021 22:20


English, 23.04.2021 22:20



Mathematics, 23.04.2021 22:20


Mathematics, 23.04.2021 22:20


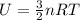
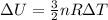
 is the change in temperature of the gas.
is the change in temperature of the gas.