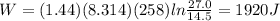Physics, 17.03.2020 04:13 jaustin1961
A 1.44-mole sample of an ideal gas is allowed to expand at a constant temperature of 258 K. The initial volume is 14.5 L and the final volume is 27.0 L. How much work does the gas perform on its container? Let the ideal-gas constant R = 8.314 J/(mol • K).
1920 J
2340 J
1550 J
1040 J

Answers: 2
Another question on Physics

Physics, 21.06.2019 14:50
Ingrid wrote the hypothesis below. if the temperature of a liquid increases, the density of the liquid decreases because the particles move farther apart. what are the variables in her hypothesis?
Answers: 3

Physics, 21.06.2019 17:10
An object is moving east, and it’s velocity changes from 65m/s to 25m/s in 10 seconds. which describes the acceleration?
Answers: 1


Physics, 22.06.2019 07:40
Which best describes how fluids change as they travel through different portions of the convection currents? they change to solids at the outer portion of the convection currents. they change to solids at the inner portion of the convection currents. they become more dense at the outer portion of the convection currents. they become more dense at the inner portion of the convection currents
Answers: 2
You know the right answer?
A 1.44-mole sample of an ideal gas is allowed to expand at a constant temperature of 258 K. The init...
Questions

Mathematics, 17.12.2020 20:10



Chemistry, 17.12.2020 20:10




Social Studies, 17.12.2020 20:10

Mathematics, 17.12.2020 20:10

Mathematics, 17.12.2020 20:10


Mathematics, 17.12.2020 20:10







Mathematics, 17.12.2020 20:10

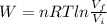
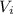 is the initial volume of the gas
is the initial volume of the gas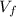 is the final volume
is the final volume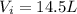 is the initial volume of the gas
is the initial volume of the gas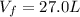 is the final volume
is the final volume