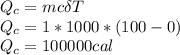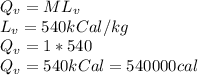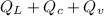The quantity of heat Q that changes the temperature L1Tof a mass mof a substance isgiven by Q = cmt:T, where c is the specific heat capacity of the substance. Forexample, forH20,c=1caljg'C",Andfora change of phase, the quantity of heat Q that changes the phase of a mass m is Q = ml., where L is the heat of fusion or heat of vaporization of the substance. For example, for H20, the heat offusion is 80 cal/g (or 80 kcaljkg) and the heat of vaporization is 540 cal/g (or 540 kcaljkg). Use these relationships to determine the number of calories to change (a) 1 kg ofO°C ice to O°C ice water, (b) 1 kg ofO°C ice water to 1 kg of 100°C boiling water, (c) 1 kg of 100°C boiling water to 1 kg of 100°C steam, and (d) 1 kg ofO°C ice to 1 kg of 100°C steam.

Answers: 3
Another question on Physics

Physics, 22.06.2019 07:00
Aball has an initial velocity of 3 m/s. if there is no friction, what is the highest it could roll?
Answers: 1

Physics, 22.06.2019 13:10
Most short-period comets do not have randomly oriented orbits because
Answers: 2

Physics, 22.06.2019 17:30
How does the entropy of steam compare to the entropy of ice?
Answers: 2

Physics, 22.06.2019 22:10
M1 = 2.8 kg, m2 = 6.72 kg, m3 = 11.2 kg, byas in .is ,is .toof m3 it 0.91 m. (in m/s)
Answers: 3
You know the right answer?
The quantity of heat Q that changes the temperature L1Tof a mass mof a substance isgiven by Q = cmt:...
Questions


Chemistry, 29.10.2019 01:31

Mathematics, 29.10.2019 01:31


Mathematics, 29.10.2019 01:31



Mathematics, 29.10.2019 01:31







Social Studies, 29.10.2019 01:31



Biology, 29.10.2019 01:31


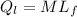 = 1 * 80
= 1 * 80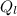 = 80 kCal = 80,000 cal
= 80 kCal = 80,000 cal