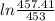Answers: 2
Another question on Physics

Physics, 22.06.2019 06:30
What are similarities and differences between refraction, reflection, diffraction and absorption?
Answers: 3

Physics, 22.06.2019 17:00
If a negatively charged particle is placed at rest in an electric potential field that increases in the positive x-direction, what will the particle do? a. accelerate in the positive x-direction b. remain at rest c. accelerate in the negative x-direction
Answers: 3


Physics, 23.06.2019 10:30
Abig box of sausages (30 kg) are lifted from the ground to the top shelf of the freezer. if the box is lifted at a constant speed, a distance of 1.75 m, what work is done by gravity?
Answers: 1
You know the right answer?
A 25-kg iron block initially at 350oC is quenched in an insulated tank that contains 100 kg of water...
Questions


Law, 20.04.2021 20:20


Physics, 20.04.2021 20:20



Chemistry, 20.04.2021 20:20

Mathematics, 20.04.2021 20:20







Mathematics, 20.04.2021 20:20





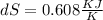
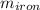 = 25 kg
= 25 kg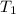 = 350°c = 623 K
= 350°c = 623 K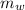 = 100 kg
= 100 kg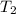 = 180°c = 453 K
= 180°c = 453 K 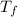
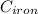 (
( 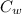 (
( 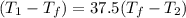
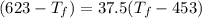
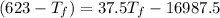
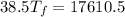
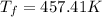
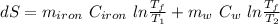
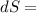 25 × 0.448 ×
25 × 0.448 × 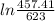 + 100 × 4.2 ×
+ 100 × 4.2 ×