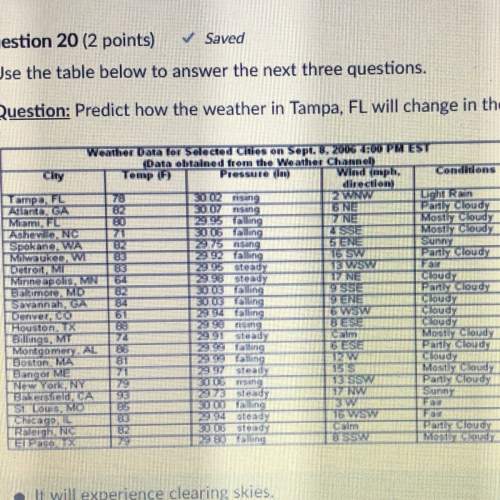
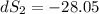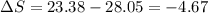One container holds 0.10 kg of water at 75 ∘C and is warmed to 95 ∘C by heating from contact with the other container. The other container, also holding 0.10 kg of water, cools from 35 ∘C to 15 ∘C. Specific heat of water is 4180 J/kg⋅∘C. Estimate the total change in entropy of two containers of water using the actual temperatures to determine the heat transferred to each container and the average temperatures to determine the change in entropy. Is this energy transfer process allowed by the first law of thermodynamics? Yes or No. is this energy transfer process allowed by the second law of thermodynamics? Yes or No

Answers: 1
Another question on Physics

Physics, 21.06.2019 13:30
Explain how plate tectonics have changed earth’s surface over time. include the role of plate tectonics in the creation of landforms.
Answers: 3

Physics, 22.06.2019 12:00
Under the action of a constant force an object accelerates at 7.8 m/s2. what will the acceleration be if (a) the force is halved? (b) the object's mass is halved? (c) the force and the object's mass are both halved? (d) the force is halved and the object's mass is doubled?
Answers: 3

Physics, 22.06.2019 12:50
Which changes would result in a decrease in the gravitational force btween two objects? check all that apply
Answers: 1

Physics, 22.06.2019 18:00
Consider an ideal gas at 27.0 degrees celsius and 1.00 atmosphere pressure. imagine the molecules to be uniformly spaced, with each molecule at the center of a small cube. what is the length l of an edge of each small cube if adjacent cubes touch but don't overlap?
Answers: 2
You know the right answer?
One container holds 0.10 kg of water at 75 ∘C and is warmed to 95 ∘C by heating from contact with th...
Questions

English, 06.11.2021 01:00

English, 06.11.2021 01:00

Computers and Technology, 06.11.2021 01:00






Mathematics, 06.11.2021 01:00


SAT, 06.11.2021 01:00


SAT, 06.11.2021 01:00

Biology, 06.11.2021 01:00

SAT, 06.11.2021 01:00





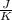
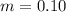 Kg
Kg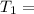 75°C
75°C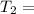 95°C
95°C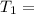 35°C
35°C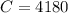
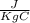
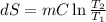
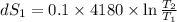
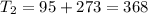 K,
K, 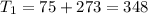 K
K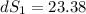
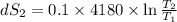
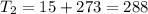 K,
K, 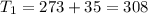 K
K