
Physics, 30.03.2020 03:53 dbn4everloved
Look at the circuit illustrated in the figure above. Assume that the values of R1 and R2 are equal. If you connect your meters test probes to points A and B in the circuit, which of the following voltages would you measure?
A. 3 V
B. 6 V
C. 0 V
D. 9 V
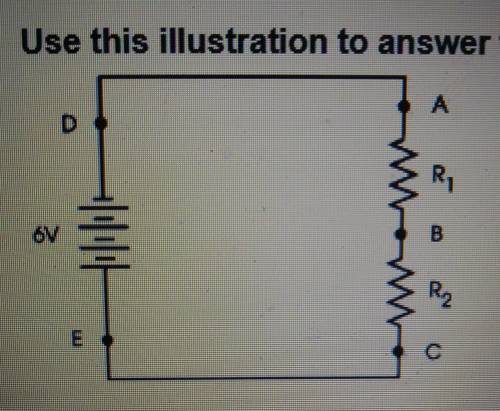

Answers: 3
Another question on Physics

Physics, 21.06.2019 17:00
These models show the electron structures of two different nonmetal elements. which element is likely more reactive, and why? element 1 is more reactive because it has fewer electron shells and is toward the top of its group on the periodic table. element 1 is more reactive because it has more electrons in its valence shell and is farther to the right on the periodic table. element 2 is more reactive because it does not have a valence shell close to the nucleus, so it will attract electrons. element 2 is more reactive because it does not have a full valence shell, so it will attract electrons.
Answers: 2

Physics, 21.06.2019 18:00
Abaseball has a mass of 0.145 kg and approaches a bat at 40.0 m/s. after it is hit, the ball leaves the bat at 50.0 m/s directly back. find the impulse of the bat on the ball. 7.25 kg*m/s 13.1 kg*m/s 5.80 kg*m/s 1.45 kg*m/s
Answers: 2

Physics, 21.06.2019 23:30
After a big snowfall, you take your favorite rocket-powered sled out to a wide field. the field is 195 m across, and you know that your sled accelerates at a rate of 3.65 m/s2 when the rocket is on. how much time will it take the sled to cross the field starting from rest, assuming the rocket is on the whole time?
Answers: 1

Physics, 22.06.2019 00:10
The energy released by a chemical reaction can be measured using a calorimeter. when barium hydroxide octahydrate crystals are reacted with dry ammonium chloride inside of a coffee cup calorimeter, the temperature of the 18.00 g of water in the calorimeter decreases from 30.0°c to 8.0°c. the equation for calculating energy absorbed or released by a reaction is: where q is the energy released or absorbed, m is the mass of water in the calorimeter, cp is the specific heat of water, and δt is the observed temperature change. if the specific heat of liquid water is 4.19 j/g·°c, how much energy was absorbed by the reaction?
Answers: 3
You know the right answer?
Look at the circuit illustrated in the figure above. Assume that the values of R1 and R2 are equal....
Questions


History, 02.10.2019 17:30




History, 02.10.2019 17:30


Mathematics, 02.10.2019 17:30

Computers and Technology, 02.10.2019 17:30

Health, 02.10.2019 17:30


Arts, 02.10.2019 17:30

Social Studies, 02.10.2019 17:30







Business, 02.10.2019 17:30



