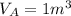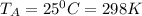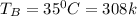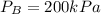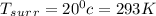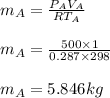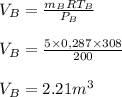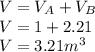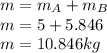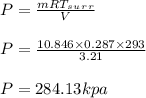Physics, 30.03.2020 17:57 twistedhyperboles
A 1-m3 tank containing air at 25 °C and 500 kPa is connected through a valve to another tank containing 5 kg of air at 35 °C and 200 kPa. Now the valve is opened, and the entire system is allowed to reach thermal equilibrium with the surroundings, which are at 20 °C. Determine the volume of the second tank and the final equilibrium pressure of air.

Answers: 2
Another question on Physics

Physics, 22.06.2019 07:00
We put a force of 50n on an object and the acceleration is 100 m/s². what is the mass of the object?
Answers: 1

Physics, 22.06.2019 09:30
For this car, predict the shape of a graph that shows distance (x) versus time (t). note that time is the independent variable and will be on the bottom axis. need asap
Answers: 1

Physics, 22.06.2019 11:00
What would be the result of an alpha particle coming into a magnetic field? a) the alpha particle will stop moving. b) the alpha particle will reverse its direction. c) the alpha particle will be deflected in a curve path. d) the alpha particle will continue to travel in a straight line.
Answers: 1

Physics, 22.06.2019 15:50
Select all the correct answers. which changes will increase the rate of reaction during combustion? decreasing the area of contact between the reactants adding more oxygen to the reaction removing heat from the reaction changing the reactants from solid form to powdered form lowering the exposure of the reactants to air
Answers: 3
You know the right answer?
A 1-m3 tank containing air at 25 °C and 500 kPa is connected through a valve to another tank contain...
Questions




English, 11.06.2020 12:57

Mathematics, 11.06.2020 12:57


Mathematics, 11.06.2020 12:57

Mathematics, 11.06.2020 12:57

Mathematics, 11.06.2020 12:57

Mathematics, 11.06.2020 12:57



Mathematics, 11.06.2020 12:57







Chemistry, 11.06.2020 12:57