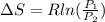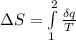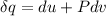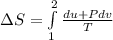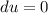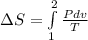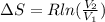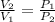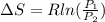A unit mass of an ideal gas at temperature T undergoes a reversible isothermal process from pressure P1 to pressure P2 while losing an amount q of heat to the surroundings at temperature T. If the gas constant of the gas is R, the entropy change of the gas âs during this process is:a. âS =R ln (P2/P1)b. âS = R ln (P1/P2)c. âS =R ln (P2/P1) - q/Td. âS = R ln (P1/P2) - q/Te. 0

Answers: 1
Another question on Physics


Physics, 22.06.2019 11:30
If the textbook weighs 19.6 newtons on venus,what is the strength of gravity on venus?
Answers: 1

Physics, 22.06.2019 14:30
What conclusion can be made based on the temperature of soil when the light hits the soil at 0°, 45°, and 90° angles in section 2 of the experiment? did your results support your hypothesis? why or why not?
Answers: 1

Physics, 22.06.2019 15:50
Select all the correct answers. which changes will increase the rate of reaction during combustion? decreasing the area of contact between the reactants adding more oxygen to the reaction removing heat from the reaction changing the reactants from solid form to powdered form lowering the exposure of the reactants to air
Answers: 3
You know the right answer?
A unit mass of an ideal gas at temperature T undergoes a reversible isothermal process from pressure...
Questions



Advanced Placement (AP), 04.05.2021 21:30



Computers and Technology, 04.05.2021 21:30




Spanish, 04.05.2021 21:30

Mathematics, 04.05.2021 21:30


History, 04.05.2021 21:30



Mathematics, 04.05.2021 21:30


Biology, 04.05.2021 21:30

Computers and Technology, 04.05.2021 21:30