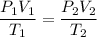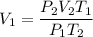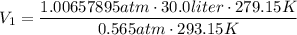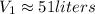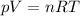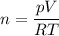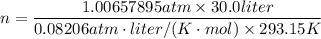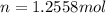Physics, 08.04.2020 16:19 angsoccer02
You are going to complete several meteorological tests using a large weather balloon. You wish to check the temperature at 3000 meters altitude. You want to make sure the balloon will not burst before it reaches that height. Some calculations are needed. The balloon manufacturer guarantees the balloon up to 47.0 Liters in size. Will the balloon make it to the necessary height without bursting? The temperature at ground level is 20 C. The pressure is 765 mmHg. The volume of the balloon prior to release is 30.0 Liters. It is filled with helium, which is lighter than air. For both calculations you will be using PV =nRT. Show all work for any credit. 1. Calculate the amount in grams of helium in the balloon. Hint remember n = moles 2. Can the balloon ascend to 3000 meters without bursting? The temperature and pressure at 3000 meters was measured at Temp = 6.0 C, Pressure = .565 atm For both calculations you will be using PV =nRT. Show all work for any credit.

Answers: 2
Another question on Physics

Physics, 22.06.2019 04:00
10 newton object is placed 3 meters from the fulcrum. at what distance on the other side would you need to place a 15 newton object to balance the lever? show your work!
Answers: 2

Physics, 22.06.2019 10:10
Which branches of natural science include the study of an organism that lived 10 million years ago
Answers: 1

Physics, 22.06.2019 15:50
Ryan is examining the energy of the particles in a bar of gold. what is ryan most likely studying?
Answers: 1

You know the right answer?
You are going to complete several meteorological tests using a large weather balloon. You wish to ch...
Questions


Advanced Placement (AP), 10.04.2021 16:10

History, 10.04.2021 16:10

Chemistry, 10.04.2021 16:10





Mathematics, 10.04.2021 16:10




Chemistry, 10.04.2021 16:10


Mathematics, 10.04.2021 16:10

Computers and Technology, 10.04.2021 16:10

English, 10.04.2021 16:10



Mathematics, 10.04.2021 16:20