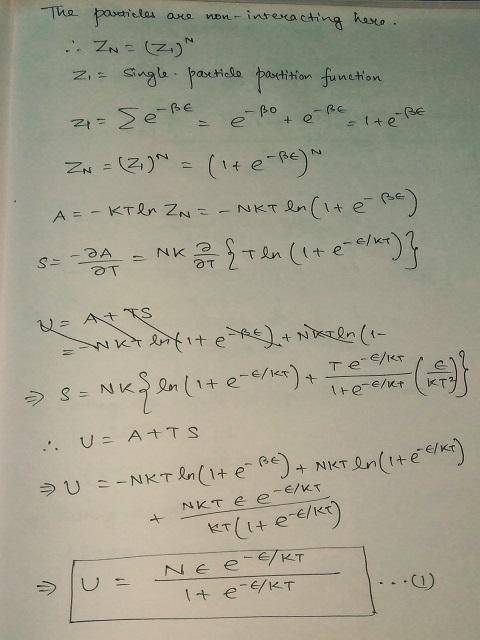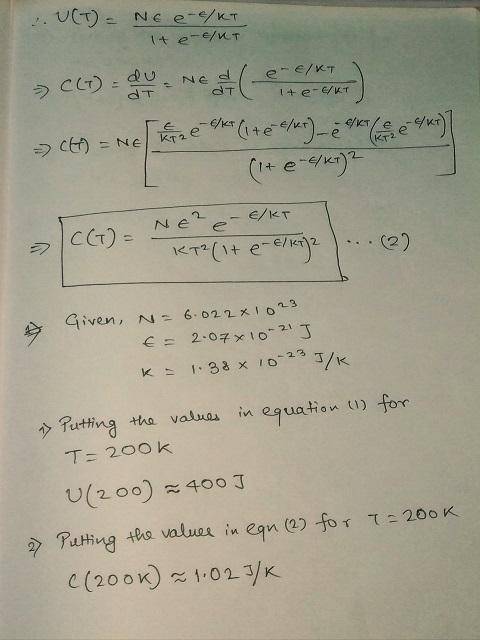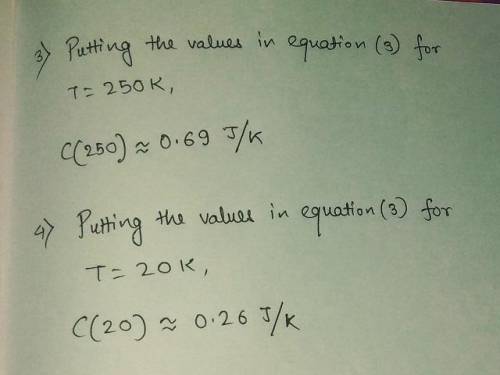Consider N two-state systems at temperature T. All systems are identical, with one state at energy 0 and the other at energy ϵ.
Using the Boltzmann factor for the two states, compute a formula for the energy U(T) as a function of N,ϵ, and T.
Using C(T)=dU/dT, derive a formula for the heat capacity in terms of the same variables.
Use the formulas for U(T) and C(T) to answer the questions below, assuming that N=6.022 × 1023 and ϵ= 2.07 × 10-21 J for all questions:
1) Compute the internal energy U when T-200 K.
2) Compute the heat capacity when T-200 K.
3) Compute the heat capacity when T-250 K.
4) Compute the heat capacity when T-20 K.

Answers: 2
Another question on Physics

Physics, 22.06.2019 00:30
Ablock of steel has a density of 0.29lbs per cubic inch. if the block has dimensions of 1 inch by 1 inch by 3 inches, what is its weight? a. 0.29lbs b. 0.58lbs c. 0.87lbs d. 1lbs
Answers: 1

Physics, 22.06.2019 10:00
Which is the correct symbol for an isotope of iodine with 53 protons and 78 neutrons?
Answers: 2


You know the right answer?
Consider N two-state systems at temperature T. All systems are identical, with one state at energy 0...
Questions








Mathematics, 20.11.2019 20:31

Mathematics, 20.11.2019 20:31

Mathematics, 20.11.2019 20:31


Chemistry, 20.11.2019 21:31




Mathematics, 20.11.2019 21:31




History, 20.11.2019 21:31