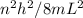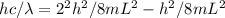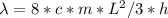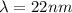A simple cyanine dye molecule has a long central carbon chain, with a total of 8 bonds, between the two nitrogen atoms. One electron per bond is essentially free to move up and down this chain. The potential energy for such an electron can be modeled as a one-dimensional box. The distance between each bond is about 0.14 nm.
What would be the transition corresponding to the lowest energy photon that this molecule could absorb? Would such a photon be in the visible range?

Answers: 2
Another question on Physics

Physics, 22.06.2019 09:00
What is a possible result of higher air temperature caused by global warming
Answers: 1

Physics, 22.06.2019 10:00
Your town is considering building a biodiesel power plant describe at least two advantages and two disadvantages
Answers: 1

Physics, 22.06.2019 11:50
The scalar triple product computes the magnitude m of the moment of a force vector f about a specified line. it is m = (r × f) · n, where r is the position vector from the line to the point of application of the force and n is a unit vector in the direction of the line. use matlab to compute the magnitude m for the case where f = [12, −5, 4] n, r = [−3, 5, 2] m, and n = [6, 5, −7].
Answers: 3

Physics, 22.06.2019 14:00
Una carga puntual de 3 x 10-6 c se coloca a 12 cm de una segunda carga puntual de - 1,5 x 10-6 c. calcular la magnitud fuerza eléctrica entre las cargas
Answers: 2
You know the right answer?
A simple cyanine dye molecule has a long central carbon chain, with a total of 8 bonds, between the...
Questions

Computers and Technology, 09.12.2021 19:40


History, 09.12.2021 19:40

English, 09.12.2021 19:40

Chemistry, 09.12.2021 19:40





Biology, 09.12.2021 19:40










Chemistry, 09.12.2021 19:40