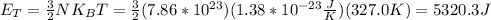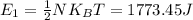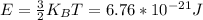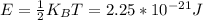A gas bottle contains 7.86×10^23 Oxygen molecules at a temperature of 327.0 K.
1. What is the...

A gas bottle contains 7.86×10^23 Oxygen molecules at a temperature of 327.0 K.
1. What is the thermal energy of the gas? (You might need to know Boltzmann's constant: kB = 1.38×10^(-23) J/K.)
2. How much energy is stored in ONE degree of freedom for the whole system?
3. What is the average energy of a single molecule?
4. On average how much energy is stored by ONE degree of freedom for ONE single molecule?

Answers: 3
Another question on Physics

Physics, 21.06.2019 17:20
Explain why the aperture of the spherical mirror should be small compared to the radius of curvature of the mirror.
Answers: 1

Physics, 22.06.2019 01:30
Question 7 [2 marks] in the circuit below, the electromotive force generated by the battery is e = 6.0 v, and the resistances are r1 = r4 = 1.0 q, r2 = r3 =2.0 q. r. the power delivered by the battery to the circuit is closest to a. 6 w в. 12 w с. 15 w d. 18 w e. 20 w final exam autumn 2014 page 5 of 9 68037 physical modelling faculty of science [2 mark] question 8 with reference to question 7, the current through resistor r2 is closest to a. 1 a в. 2 а c. 3 a d. 4 a e. 6 a
Answers: 2


Physics, 22.06.2019 18:30
En un laboratorio, nakisha mezcla una solución de hidróxido de sodio con un indicador llamado fenolftaleína. cuando se combinan, crean una solución de color rosa. nakisha se pregunta si la mezcla de otras soluciones con fenolftaleína también creará este color rosa. ? cómo podría nakisha utilizar el proceso de investigación científica para determinar si la mezcla de otras soluciones con fenolftaleína también creará un color rosa? marque las que correspondan.
Answers: 2
You know the right answer?
Questions

Mathematics, 19.02.2021 21:40



English, 19.02.2021 21:40

Mathematics, 19.02.2021 21:40



Mathematics, 19.02.2021 21:40




Mathematics, 19.02.2021 21:50

Mathematics, 19.02.2021 21:50

Mathematics, 19.02.2021 21:50


English, 19.02.2021 21:50


Biology, 19.02.2021 21:50

Biology, 19.02.2021 21:50