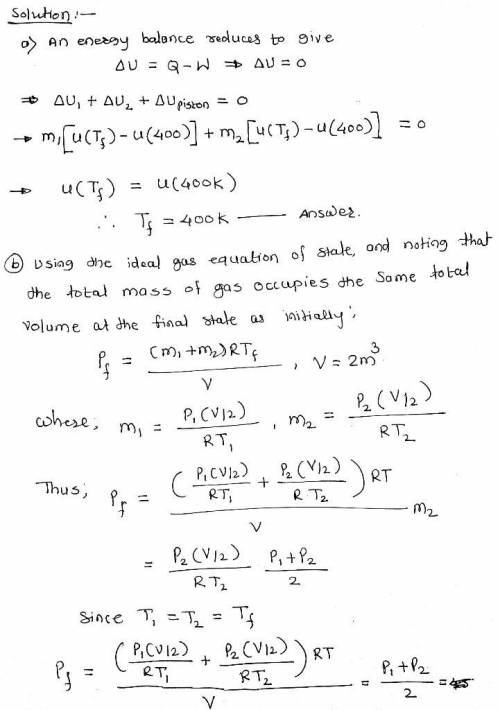
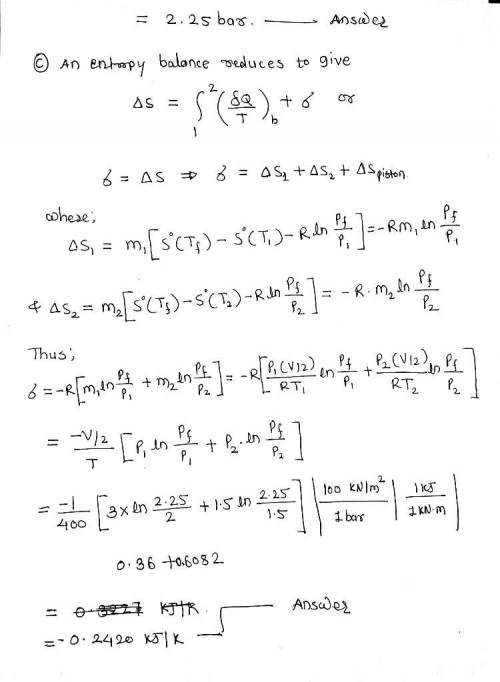
Of the piston is 1.0 m3 of air at 400 K, 3 bar. On the other side is 1.0 m3 of air at 400 K, 1.5 bar. The piston is released and equilibrium is attained, with the piston experiencing no change of state. Employing the ideal gas model for the air, determine a. the final temperature of the air, in K. b. the final pressure of the air, in bar. c. the amount of entropy produced, in kJ/K

Answers: 2
Another question on Physics

Physics, 22.06.2019 06:50
Much of the information taught in earth-space science would not be available if it weren’t for our remote sensing abilities. explain two ways our remote sensing strategies contribute to our knowledge of these topics.
Answers: 3

Physics, 22.06.2019 08:30
What properties of a moving object are used in determining the object's energy of motion
Answers: 2


Physics, 22.06.2019 17:00
Sawyer is studying diffraction. he draws a diagram of a plane wave to show how light waves travel. which best describes sawyer’s error? the wave fronts should be perpendicular to the direction in which the waves move. the arrow showing the direction of movement of the waves should be pointing to the left. the arrow showing the direction of movement of the waves should be pointing downward. the wave fronts should be both parallel and perpendicular to the direction in which the waves move.
Answers: 3
You know the right answer?
Of the piston is 1.0 m3 of air at 400 K, 3 bar. On the other side is 1.0 m3 of air at 400 K, 1.5 bar...
Questions

Mathematics, 20.09.2020 01:01


Mathematics, 20.09.2020 01:01


Mathematics, 20.09.2020 01:01




Social Studies, 20.09.2020 01:01

History, 20.09.2020 01:01


Medicine, 20.09.2020 01:01


Social Studies, 20.09.2020 01:01

Mathematics, 20.09.2020 01:01


Biology, 20.09.2020 01:01


Biology, 20.09.2020 01:01