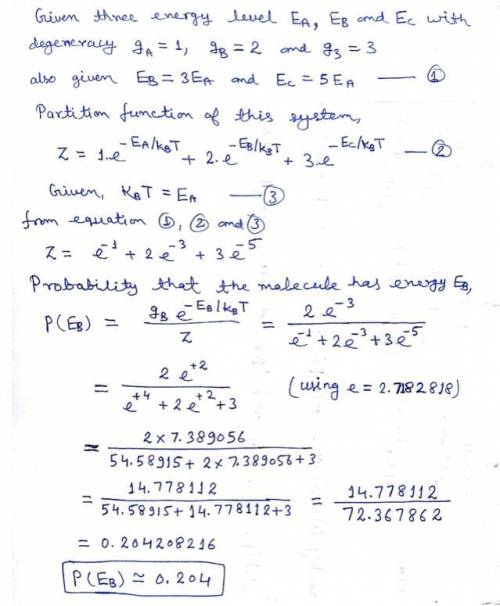
Physics, 16.04.2020 01:07 thomasmurphy200
More than one distinct microstate of a system can have a particular energy (a macrostate). The purpose of this interactive example is to ensure that you know how to deal with this fact when calculating the probability that a small system (here, a molecule) in contact with a thermal reservoir has a particular energy. Let's consider a molecule that can be in one of six microstates. It exchanges energy with a thermal reservoir at a temperature T. One microstate has energy EA, two states have energy EB = 3EA, and three states have energy EC = 5EA. To simplify the numerical calculation, you may assume that the temperature is such that kBT = EA. What is the probability that the molecule has energy EB? P(EB) =

Answers: 3
Another question on Physics

Physics, 22.06.2019 09:10
The diagram shows a series of volcanic island and a hot spot determine the direction of movement of the tectonic plate that for the island
Answers: 2

Physics, 22.06.2019 11:00
1. jay fills a wagon with sand (about 20 kg) and pulls it with a rope 30 m along the beach. he holds the rope 25° above the horizontal. the rope exerts a 20-n tension force on the wagon. how much work does the rope do on the wagon?
Answers: 1

Physics, 22.06.2019 12:30
Urgent pls a. coal consumption levels off and remains flat. b. petroleum, natural gas, and renewables show an increase in consumption c. more nonrenewable resources continued to be consumed than renewable. d. there is little projected increase in nuclear energy use. e. carbon dioxide emissions are projected to decline as we approach 2040. global energy consumption is defined as the total energy used by an individual or organizations from around the world. use the graph above to analyze the projected energy consumption from now until 2040. which statements in the prompt apply? a) a, b, d b) b, c, d c) a, c, d d) a, b, c, d
Answers: 1

Physics, 22.06.2019 14:40
According to valence bond theory, which orbitals overlap in the formation of the bond in hf according to valence bond theory, which orbitals overlap in the formation of the bond in hf 2s on h and 2p on f 1s on h and 2s on f 1s on h and 1p on f 1s on h and 2p on f 1s on h and 3p on f
Answers: 3
You know the right answer?
More than one distinct microstate of a system can have a particular energy (a macrostate). The purpo...
Questions




History, 10.09.2021 19:20






English, 10.09.2021 19:20


Social Studies, 10.09.2021 19:20


Mathematics, 10.09.2021 19:30



Mathematics, 10.09.2021 19:30

Mathematics, 10.09.2021 19:30