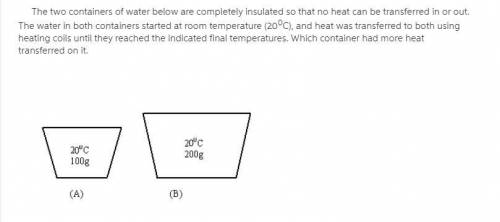
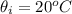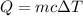The two containers of water below are completely insulated so that no heat can be transferred in or out. The water in both containers started at room temperature (20 0C), and heat was transferred to both using heating coils until they reached the indicated final temperatures.
Which container had more heat transferred on it.?

Answers: 2
Another question on Physics

Physics, 22.06.2019 04:30
The graph describes the motion of an object. the object moves with from a to b. it from b to c. it moves with from c to d.
Answers: 1

Physics, 22.06.2019 13:00
Which of the following correctly describes what happens when an atomic bomb explodes? small pieces of fissionable material are joined and form a body with a mass greater than the critical mass, the relative number of neutrons escaping decreases, and a chain reaction and explosion result. large pieces of fissionable matter are brought together quickly and form a body with a mass smaller than the critical mass, the relative number of escaping neutrons increases, and a chain reaction and explosion result.
Answers: 2

Physics, 23.06.2019 00:10
During cooling, the kinetic energy of the molecules falls. why does this happen? a.the motion of the molecules increases. b. the motion of the molecules slows down. c.the van der waals forces between molecules decrease d. the forces of attraction between the molecules are overcome.
Answers: 2

Physics, 23.06.2019 07:00
Can someone me with my physics questions? i need correct answers only!
Answers: 2
You know the right answer?
The two containers of water below are completely insulated so that no heat can be transferred in or...
Questions

History, 20.10.2020 19:01



Mathematics, 20.10.2020 19:01



History, 20.10.2020 19:01


English, 20.10.2020 19:01





Biology, 20.10.2020 19:01

Mathematics, 20.10.2020 19:01

Chemistry, 20.10.2020 19:01

Computers and Technology, 20.10.2020 19:01


Chemistry, 20.10.2020 19:01

Mathematics, 20.10.2020 19:01