
In Parts B and C you saw that, according to Bohr's postulate, the electron radius r and the electron velocity v only have certain allowable values. Plug the values obtained for these two quantities into the energy statement given below (E=) to arrive at a new statement for the allowed energy levels in the Bohr atom. Express answer in terms of e, m, n, h, \epsilon _{0} . Equations:
v=nh/2mr\pi
r=n^{2}h^{2}\epsilon _{0}/m\pi e^{2}
E=\frac{1}{2}mv^{2}-(e^{2}/4\pi r\epsilon _{0})

Answers: 2
Another question on Physics

Physics, 22.06.2019 07:00
The cerebellum controls? thirst sensations sleep-wake cycles hand-eye coordination heart rate and pulse
Answers: 1

Physics, 22.06.2019 09:00
The pressure proportional to the area a- inversely b- directly c- increase d-decrease
Answers: 2

Physics, 22.06.2019 09:00
One form of energy can be another type of energy. a. created to form b. transformed into c. destroyed and then created to form
Answers: 1

Physics, 22.06.2019 14:00
Me pl give an example of a collision in real life. use the law of conservation of energy to describe the transfer of momentum. be sure and discuss the momentum before and after the collision occurs. you will need at least 3 sentences to thoroughly answer this question.
Answers: 3
You know the right answer?
In Parts B and C you saw that, according to Bohr's postulate, the electron radius r and the electron...
Questions


History, 05.05.2020 20:19






Mathematics, 05.05.2020 20:19

Mathematics, 05.05.2020 20:19

SAT, 05.05.2020 20:19


Mathematics, 05.05.2020 20:19








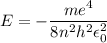
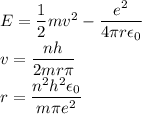
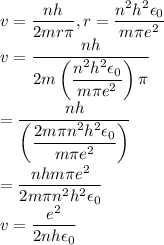
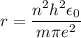
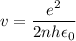
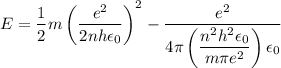
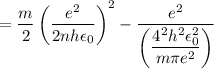
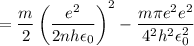
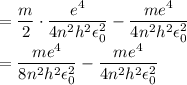
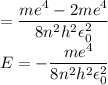
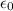 is:
is:

