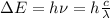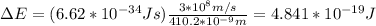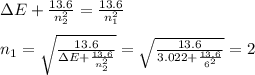Physics, 05.05.2020 16:20 milkshakegrande101
An electron in the n = 6 level emits a photon with a wavelength of 410.2 nm. to what energy level does the electron move?
a. n = 1
b. n = 2
c. n = 3
d. n = 4
e. n = 5

Answers: 2
Another question on Physics

Physics, 22.06.2019 07:00
Photoelectrons with a maximum speed of 6.50 x 107 m/s are ejected from a surface in the presence of light with a frequency of 6.75 x 1014hz. if the mass of an electron is 9.10 x 10-31 kg, calculate in joules the maximum kinetic energy of a single electron. 3.84 x 10-15 j 1.92 x 10-15 j 5.92 x 10-23 j 3.07 x 10-16 j
Answers: 1

Physics, 22.06.2019 16:50
Which best describes the first law of thermodynamics as compared to the second law of thermodynamics? a. the first law describes how thermal energy is conserved but not the direction it moves. b. the first law describes the direction thermal energy moves but not how it is conserved. c. the first law describes how thermal energy can be created but not how it can be destroyed. d. the first law describes how thermal energy can be destroyed but not how it can be created.
Answers: 1

Physics, 22.06.2019 18:10
A200-n force is applied to the foot-operated air pump. the return spring s exerts a 2.6-n·m moment on member oba for this position. determine the corresponding compression force c in the cylinder bd. if the diameter of the piston in the cylinder is 40 mm, estimate the air pressure generated for these conditions. state any assumptions. enter a positive number for the compression force c.
Answers: 2

Physics, 23.06.2019 00:00
Based on this activity, is kinetic energy always transformed into potential energy?
Answers: 1
You know the right answer?
An electron in the n = 6 level emits a photon with a wavelength of 410.2 nm. to what energy level do...
Questions

Arts, 17.11.2020 21:10

Mathematics, 17.11.2020 21:10

Physics, 17.11.2020 21:10

Mathematics, 17.11.2020 21:10

Mathematics, 17.11.2020 21:10



Mathematics, 17.11.2020 21:10

Social Studies, 17.11.2020 21:10


Social Studies, 17.11.2020 21:10

History, 17.11.2020 21:10

Arts, 17.11.2020 21:10





Social Studies, 17.11.2020 21:10

Social Studies, 17.11.2020 21:10

Mathematics, 17.11.2020 21:10

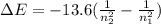 (1)
(1)