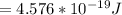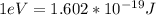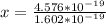Physics, 05.05.2020 05:58 NotYourStudent
6) The electron volt (eV) is a convenient unit of energy for expressing atomic-scale energies. It is the amount of energy that an electron gains when subjected to a potential of 1 volt; 1 eV = 1.602 × 10–19 J. Using the Bohr model, determine the energy, in electron volts, of the photon produced when an electron in a hydrogen atom moves from the orbit with n = 5 to the orbit with n = 2. Show your calculations.

Answers: 3
Another question on Physics

Physics, 21.06.2019 22:30
Select the word from the list that best fits the definition classification of most stars on the h-r diagram
Answers: 1

Physics, 22.06.2019 13:30
The period of a pendulum varies directly as the square root of the length of the pendulum and inversely as the square root of the acceleration due to gravity. find the period when the length is 144 cm and the acceleration due to gravity is 980 cm per second squared, if the period is 7pi seconds when the length is 289 cm and the acceleration due to gravity is 980 cm per second squared.
Answers: 2

Physics, 22.06.2019 14:20
What are the starting materials for nuclear fission? two small nuclei two large nuclei a neutron and a large nucleus a neutron and a small nucleus
Answers: 2

You know the right answer?
6) The electron volt (eV) is a convenient unit of energy for expressing atomic-scale energies. It is...
Questions







Mathematics, 05.08.2021 23:30

Mathematics, 05.08.2021 23:30


Mathematics, 05.08.2021 23:30








Chemistry, 05.08.2021 23:40


History, 05.08.2021 23:40

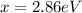
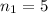
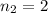
![\Delta E = k [\frac{1}{n^2 _1} + \frac{1}{n^2 _2} ]](/tpl/images/0633/9061/88f65.png)
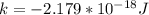
![\Delta E = - 2.179 * 10^{-18} [\frac{1}{5^2 _1} + \frac{1}{2^2 _2} ]](/tpl/images/0633/9061/06bdc.png)