A sample of 2.50 kg of water is held at a temperature of 100°C. How much
energy must be added...

Physics, 06.05.2020 00:46 Pranav2004
A sample of 2.50 kg of water is held at a temperature of 100°C. How much
energy must be added to completely turn the liquid water to water vapor?
(The latent heat of vaporization for water is 2260 kJ/kg; the latent heat of
fusion for water is 333 kJ/kg.)
O
A. 5650 kJ
O B. 133 kJ
O C. 904 kg
O D. 833 kJ

Answers: 1
Another question on Physics

Physics, 21.06.2019 20:30
Asmall metal bar, whose initial temperature was 10° c, is dropped into a large container of boiling water. how long will it take the bar to reach 70° c if it is known that its temperature increases 2° during the first second? (the boiling temperature for water is 100° c. round your answer to one decimal place.) sec how long will it take the bar to reach 98° c? (round your answer to one decimal place.)
Answers: 2

Physics, 22.06.2019 02:30
Which of the following is not am example of a polymer? protein nylon kevlar concreta
Answers: 1

Physics, 22.06.2019 06:30
Suppose you have a box. which of these is not necessarily a true statement regarding the relationship between the force applied to the box, the work done on the box, and the distance the box is moved? a. if the box is not moved, no force is being applied to the box. b. if no force is applied to the box, no work is done on the box. c. if work is done on the box, then a force is applied to the box. d. if work is done on the box, then the box is moved a distance.
Answers: 2

Physics, 22.06.2019 14:00
Me pl give an example of a collision in real life. use the law of conservation of energy to describe the transfer of momentum. be sure and discuss the momentum before and after the collision occurs. you will need at least 3 sentences to thoroughly answer this question.
Answers: 3
You know the right answer?
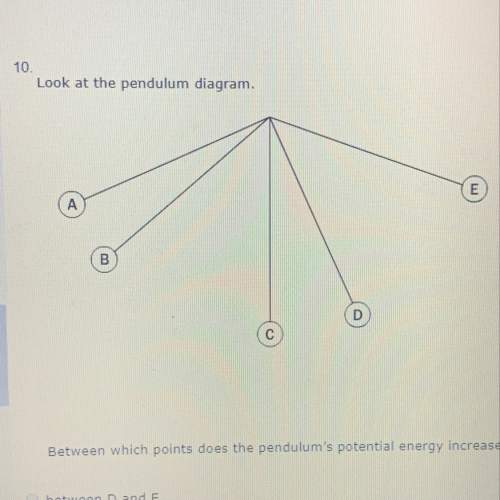
Questions





Geography, 24.03.2020 21:42



Mathematics, 24.03.2020 21:42

History, 24.03.2020 21:42


History, 24.03.2020 21:42





Computers and Technology, 24.03.2020 21:42



Mathematics, 24.03.2020 21:42

Mathematics, 24.03.2020 21:42