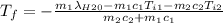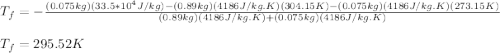Physics, 06.05.2020 04:44 Artemis3821
A thermos contains m1 = 0.89 kg of tea at T1 = 31° C. Ice (m2 = 0.075 kg, T2 = 0° C) is added to it. The heat capacity of both water and tea is c = 4186 J/(kg⋅K), and the latent heat of fusion for water is Lf = 33.5 × 104 J/kg. show answer No Attempt 50% Part (a) Input an expression for the final temperature after the ice has melted and the system has reached thermal equilibrium. Part (b) What is the final temperature in Kelvin?

Answers: 2
Another question on Physics

Physics, 22.06.2019 07:20
If 2 moles of co_2 at 2l and 500k are expanded reversibly to 20l, more work can be obtained from an adiabatic process than from an isothermal process. is the above statement true or false?
Answers: 3

Physics, 22.06.2019 12:00
You have a resistor and a capacitor of unknown values. first, you charge the capacitor and discharge it through the resistor. by monitoring the capacitor voltage on an oscilloscope, you see that the voltage decays to half its initial value in 2.70 miss . you then use the resistor and capacitor to make a low-pass filter. what is the crossover frequency fc?
Answers: 2

Physics, 22.06.2019 17:00
Using proper grammar, spelling, and punctuation, write at least one 5 sentence paragraph describing 3 ways we use the elements of the electromagnetic spectrum (ems) in our everyday lives.
Answers: 2

Physics, 22.06.2019 19:30
Which describes increasing the efficiency of energy resource
Answers: 1
You know the right answer?
A thermos contains m1 = 0.89 kg of tea at T1 = 31° C. Ice (m2 = 0.075 kg, T2 = 0° C) is added to it....
Questions







Social Studies, 23.06.2020 00:57


History, 23.06.2020 00:57








Biology, 23.06.2020 00:57



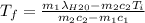
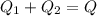
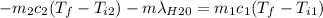 ( 1 )
( 1 )