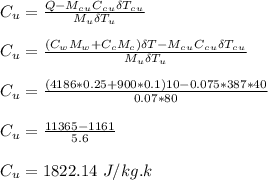Physics, 06.05.2020 16:01 brandonleestewovilgw
An aluminum calorimeter with a mass of 100 g contains 250 g of water. The calorimeter and water are in thermal equilibrium at 10.0°C. Two metallic blocks are placed into the water. One is a 75.0-g piece of copper at 60.0°C. The other has a mass of 70.0 g and is originally at a temperature of 100°C. The entire system stabilizes at a final temperature of 20.0°C.
Determine the specific heat of the unknown sample.

Answers: 1
Another question on Physics

Physics, 22.06.2019 10:30
Arunner is training for an upcoming marathon by running around a 100-m-diameter circular track at constant speed. let a coordinate system have its origin at the center of the circle with the x-axis pointing east and the y-axis north. the runner starts at (x, y) = (50 m, 0 m) and runs 2.5 times around the track in a clockwise direction.
Answers: 1

Physics, 22.06.2019 17:30
Aparticle moves in a circle 1.50 m in radius . through what angle in radians does it rotate if it moves through an arc length of 2.50m? what is the angle in degrees?
Answers: 1

Physics, 22.06.2019 20:00
Using the free-body diagram, calculate the net force acting on the sled. is the sled in a state of dynamic equilibrium?
Answers: 3

Physics, 22.06.2019 20:00
Awave has a wavelength of 7 mm and a frequency of 19 hertz. what is its speed?
Answers: 1
You know the right answer?
An aluminum calorimeter with a mass of 100 g contains 250 g of water. The calorimeter and water are...
Questions

Business, 18.10.2019 09:50


Mathematics, 18.10.2019 09:50

Mathematics, 18.10.2019 09:50








Mathematics, 18.10.2019 09:50

Chemistry, 18.10.2019 09:50


Mathematics, 18.10.2019 09:50

Mathematics, 18.10.2019 09:50


History, 18.10.2019 09:50

Mathematics, 18.10.2019 09:50

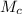 = 100 g
= 100 g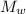 = 250 g
= 250 g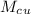 = 75 g
= 75 g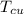 = 60.0°C
= 60.0°C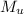 = 70.0 g
= 70.0 g 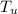 = 100°C.
= 100°C.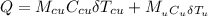
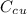 is the specific heat capacity of copper
is the specific heat capacity of copper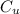 is the specific heat capacity of unknown sample
is the specific heat capacity of unknown sample