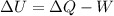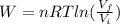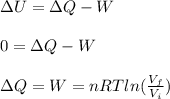An ideal monatomic gas at temperature T is held in a container. If the gas is compressed isothermally, that is at constant temperature, from a volume of Vi to Vf ,
a) What is the change in the (internal) energy of the gas?
b) How much work has been done on the gas?
c) Has heat been transferred into or out of the gas during the process? If so, how much?
d) Show that the 1st law of thermodynamics is satisfied.

Answers: 3
Another question on Physics

Physics, 22.06.2019 16:40
Panel bc in fig. p2.76 is semi-circular, with the 3 meter radius and horizontal straight edge through point b. compute (a) the hydrostatic force of the water on the panel, (b) its center of pressure, and (c) the moment of this force about point b. assume atmospheric pressure on the dry side of the panel
Answers: 3

Physics, 22.06.2019 18:30
Asmall grinding wheel has a moment of inertia of 4.0×10−5 kg⋅m2 . what net torque must be applied to the wheel for its angular acceleration to be 150 rad/s2 ?
Answers: 3


Physics, 23.06.2019 07:30
The electric field inside a battery generates an electric potential difference of 12v. the plates are seperated by a distance of 0.25m. what strength of the electric field?
Answers: 3
You know the right answer?
An ideal monatomic gas at temperature T is held in a container. If the gas is compressed isothermall...
Questions








History, 27.07.2019 07:00


Biology, 27.07.2019 07:00

Social Studies, 27.07.2019 07:00

History, 27.07.2019 07:00






Chemistry, 27.07.2019 07:00