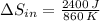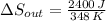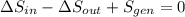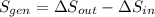Physics, 12.06.2020 01:57 itisonlyluis
When an aluminum bar is connected between a hot reservoir at 860 K and a cold reservoir at 348 K, 2.40 kJ of energy is transferred by heat from the hot reservoir to the cold reservoir
(a) In this irreversible process, calculate the change in entropy of the hot reservoir.
J/K
(b) In this irreversible process, calculate the change in entropy of the cold reservoir.
J/K
(c) In this irreversible process, calculate the change in entropy of the Universe, neglecting any change in entropy of the aluminum rod.
J/K
(d) Mathematically, why did the result for the Universe in part (c) have to be positive?

Answers: 3
Another question on Physics

Physics, 22.06.2019 00:00
Astate of matter all that has define volume and can flow is a(n)
Answers: 3

Physics, 22.06.2019 07:40
Which best describes how fluids change as they travel through different portions of the convection currents? they change to solids at the outer portion of the convection currents. they change to solids at the inner portion of the convection currents. they become more dense at the outer portion of the convection currents. they become more dense at the inner portion of the convection currents
Answers: 2


Physics, 23.06.2019 00:00
What energy transformations occur each time the skateboarder rolls up the ramp?
Answers: 2
You know the right answer?
When an aluminum bar is connected between a hot reservoir at 860 K and a cold reservoir at 348 K, 2....
Questions

English, 04.05.2021 18:20


Social Studies, 04.05.2021 18:20


Mathematics, 04.05.2021 18:20








Mathematics, 04.05.2021 18:20



Mathematics, 04.05.2021 18:20

Chemistry, 04.05.2021 18:20



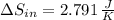 , b)
, b) 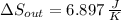 , c)
, c) 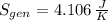 , d) Due to irreversibilities due to temperature differences.
, d) Due to irreversibilities due to temperature differences.