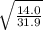Physics, 18.06.2020 18:57 SupremeNerdx
Fill in the blanks for the following: Arigid container of volume 100.0 Liters contains Oxygen gas. It is at room temperature (293 Kelvin), and is at atmospheric pressure (absolute pressure, meaning the gauge pressure is zero). Therefore, the number of moles of Oxygen molecules . Also, the rms-velocity inside is of these Oxygen molecules is most nearly , which is the rms-speed of the Nitrogen molecules just outside the container (the rigid container and its surroundings are in thermal equilibrium).

Answers: 3
Another question on Physics

Physics, 22.06.2019 05:30
Agas expands from an initial volume of 0.040 m^3 and an initial pressure of 210 kpa to a final volume of 0.065 m^3 while its temperature is kept constant. how much work is done by the system?
Answers: 1

Physics, 22.06.2019 07:30
The slope of a velocity time graph over any interval of time gives the during that interval
Answers: 2

Physics, 22.06.2019 09:00
One form of energy can be another type of energy. a. created to form b. transformed into c. destroyed and then created to form
Answers: 1

Physics, 22.06.2019 09:30
(**i would really appreciate if this was answered soon** ) which pair of quantities includes one quantity that increases as the other decreases during simple harmonic motion?
Answers: 3
You know the right answer?
Fill in the blanks for the following:
Arigid container of volume 100.0 Liters contains Oxygen gas....
Questions



English, 24.08.2019 12:00

Spanish, 24.08.2019 12:00

Social Studies, 24.08.2019 12:00

Physics, 24.08.2019 12:00

Chemistry, 24.08.2019 12:00

History, 24.08.2019 12:00


Physics, 24.08.2019 12:00

Biology, 24.08.2019 12:00


Biology, 24.08.2019 12:00

Physics, 24.08.2019 12:00


Social Studies, 24.08.2019 12:00

Social Studies, 24.08.2019 12:00

Social Studies, 24.08.2019 12:00


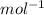

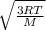
 = 478.6 m/s
= 478.6 m/s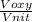 =
= 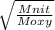
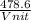 =
=