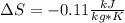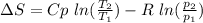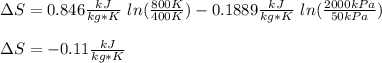Physics, 14.07.2020 17:01 oneicyahdaley10
Carbon dioxide initially at 50 kPa, 400 K, undergoes a process in a closed system until its pressure and temperature are 2 MPa and 800 K, respectively. Assuming an ideal gas behaviour, find the entropy change of the carbon dioxide by assuming that the specific heats are constant. For the gas, take Cp = 0.846 kJ/kg. K and R = 0.1889 kJ/kg. K

Answers: 3
Another question on Physics

Physics, 22.06.2019 03:00
Which of the following harmful chemicals are found in tobacco smoke? a. carbon monoxide b. carbon dioxide c. nicotine b. carbon dioxide d. both a and c
Answers: 2

Physics, 22.06.2019 08:40
Apulley system is used to lift a 2,000 newton engine up a distance of 3 meters. the operator must apply a force of 250 newtons to the chain of the pulley system to lift the motor. to lift the engine 3 meters, the operator must pull a total of 30 meters of chain through the pulley system. what is the value of di?
Answers: 1

Physics, 22.06.2019 11:00
Looking at this barometer, is the air pressure high or low? what type of weather would you expect? high, bad low, bad high, good low, good
Answers: 1

You know the right answer?
Carbon dioxide initially at 50 kPa, 400 K, undergoes a process in a closed system until its pressure...
Questions


Mathematics, 17.03.2021 23:50


Physics, 17.03.2021 23:50

Mathematics, 17.03.2021 23:50


Mathematics, 17.03.2021 23:50



Business, 17.03.2021 23:50





Mathematics, 17.03.2021 23:50


Computers and Technology, 17.03.2021 23:50

History, 17.03.2021 23:50

Physics, 17.03.2021 23:50

Mathematics, 17.03.2021 23:50