
Physics, 30.07.2020 01:01 santosbeti90
Salt compounds are commonly used to melt ice that forms on sidewalks in the winter. A common chemical that is used to melt sidewalk ice is calcium chloride, CaCl2(s). When calcium chloride dissolves into solution is releases thermal energy which aides in melting
the ice.
The molar mass of water is 18.02 g/mol
The thermal energy, in kilojoules (kJ) that must be released from the calcium chloride,
CaCl2(s), to melt 10.0 kg of ice, expressed in scientific notation is a. bc x 104 k).
46
55
The values of a, b, c, and d.

Answers: 2
Another question on Physics

Physics, 21.06.2019 16:30
You throw a baseball directly upward at time t = 0 at an initial speed of 12.3 m/s. what is the maximum height the ball reaches above where it leaves your hand? at what times does the ball pass through half the maximum height? ignore air resistance and take g = 9.80 m/s2.
Answers: 1

Physics, 22.06.2019 13:00
Ways that industry and agriculture use physical properties to separate substances
Answers: 1

Physics, 22.06.2019 17:30
Chameleons catch insects with their tongues, which they can rapidly extend to great lengths. in a typical strike, the chameleon's tongue accelerates at a remarkable 220 m/s2 for 20 ms, then travels at constant speed for another 30 ms.
Answers: 1

You know the right answer?
Salt compounds are commonly used to melt ice that forms on sidewalks in the winter. A common chemica...
Questions






English, 27.11.2020 04:30





Mathematics, 27.11.2020 04:30


Business, 27.11.2020 04:30







Social Studies, 27.11.2020 04:30

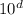 kJ
kJ

