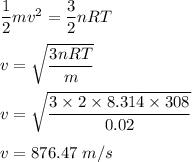
Physics, 12.08.2020 04:01 jaidencoolman2510
What is the average velocity of atoms in 2.00 mol of neon (a monatomic gas)
at 308 K? Use the equation: -mv2
2
For m, use 0.02000 kg. Remember that R = 8.31 J/(mol-K).
= ER
3
2
nRT
A. 1540 m/s
B. 876 m/s
C. 87.6 m/s
O D. 15,400 m/s

Answers: 1
Another question on Physics

Physics, 22.06.2019 01:00
Complete the sentence to describe the law of conservation of energy. the law of conservation of energy states that energy cannot be created or , but it can be
Answers: 1

Physics, 22.06.2019 17:50
If there's a small amount of friction between two surfaces, the result could be select all that applya. no movement b. heatc. a little bit of movementd. sliding around
Answers: 2

Physics, 23.06.2019 02:00
How do you count the total number of atoms present on one side of a chemical equation?
Answers: 1

Physics, 23.06.2019 03:00
The stopwatch used by a student to measure velocity of a pulse in a slinky was of least count 0.1 second. he stops the stopwatch when a pulse has made 3 journeys from one end to the other of the slinky. he finds the seconds hand to be at 52nd division. calculate the correct time noted by him.
Answers: 3
You know the right answer?
What is the average velocity of atoms in 2.00 mol of neon (a monatomic gas)
at 308 K? Use the equat...
Questions







Mathematics, 21.02.2020 01:47








Mathematics, 21.02.2020 01:49


Biology, 21.02.2020 01:49