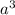Physics, 19.08.2020 01:01 chandranewlon
The atomic mass number of copper is A=64. Assume that atoms in solid copper form a cubic crystal lattice. To envision this, imagine that you place atoms at the centers of tiny sugar cubes, then stack the little sugar cubes to form a big cube. If you dissolve the sugar, the atoms left behind are in a cubic crystal lattice. What is the smallest distance between two copper atoms?

Answers: 3
Another question on Physics

Physics, 22.06.2019 12:50
The heliocentric and the geocentric models of the solar system included these central principles
Answers: 1

Physics, 22.06.2019 16:00
While the change in blank will remain the same during a collision, the force needed to bring an object to a stop can be blank if the time if collision is blank
Answers: 1

Physics, 23.06.2019 00:30
Which of the following is a true statement about the acceleration ~a of the pendulum bob ~a is equal to the acceleration due to gravity. ~a is perpendicular to the bob’s trajectory. ~a is equal to the instantaneous rate of change in velocity. ~a is tangent to the bob’s trajectory.
Answers: 1

Physics, 23.06.2019 06:30
Acopper sheet has a volume of 100 cm³. if the density of copper is 8.9 g/cm³, what is the mass of the sheet? a. 11 g b. 8.9 g c. 189 g d. 890 g
Answers: 1
You know the right answer?
The atomic mass number of copper is A=64. Assume that atoms in solid copper form a cubic crystal lat...
Questions


Biology, 21.11.2019 23:31

English, 21.11.2019 23:31








Spanish, 21.11.2019 23:31


Mathematics, 21.11.2019 23:31


Mathematics, 21.11.2019 23:31