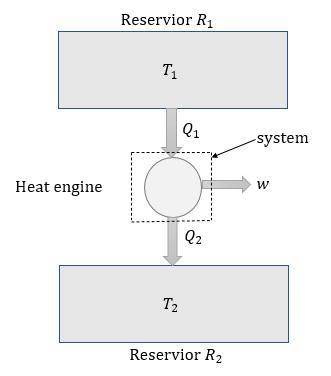
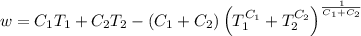A reversible heat engine, operating in a cycle, withdraws thermal energy from a high-temperature reservoir (the temperature of which consequently decreases), performs work w, and rejects thermal energy into a low-temperature reservoir (the temperature of which consequently increases). The two reservoirs are, initially, at the temperatures T1 and T2 and have constant heat capacities C1 and C2, respectively. Calculate the final temperature of the system and the maximum amount of work which can be obtained from the engine.

Answers: 2
Another question on Physics

Physics, 22.06.2019 14:00
Ascientific blank must be testable and capable of being proven false?
Answers: 1

Physics, 22.06.2019 14:50
Nitrogen (n2) undergoes an internally reversible process from 6 bar, 247°c during which pν1.2 = constant. the initial volume is 0.1 m3 and the work for the process is 121.14 kj. assuming ideal gas behavior, and neglecting kinetic and potential energy effects, determine heat transfer, in kj, and the entropy change, in kj/s. show the process on a t-s diagram.
Answers: 2

Physics, 22.06.2019 18:30
Which word expression describes how much to calculate pressure
Answers: 1

Physics, 22.06.2019 19:20
Two kilograms of air within a piston–cylinder assembly executes a carnot power cycle with maximum and minimum temperatures of 800 k and 300 k, respectively. the heat transfer to the air during the isothermal expansion is 60 kj. at the end of the isothermal expansion the volume is 0.4 m3. assume the ideal gas model for the air. determine the thermal efficiency, the volume at the beginning of the isothermal expansion, in m3, and the work during the adiabatic expansion, in kj.
Answers: 1
You know the right answer?
A reversible heat engine, operating in a cycle, withdraws thermal energy from a high-temperature res...
Questions

English, 13.07.2019 08:30

English, 13.07.2019 08:30

Mathematics, 13.07.2019 08:30

Mathematics, 13.07.2019 08:30




Mathematics, 13.07.2019 08:30

English, 13.07.2019 08:30


Mathematics, 13.07.2019 08:30


Geography, 13.07.2019 08:30

Mathematics, 13.07.2019 08:30

Mathematics, 13.07.2019 08:30




Mathematics, 13.07.2019 08:30

Geography, 13.07.2019 08:30

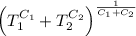
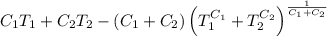 .
.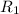 is the reservior having temperature
is the reservior having temperature 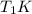 and heat capicity
and heat capicity 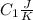 and
and 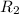 is the reservior having temperature
is the reservior having temperature 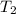 and heat capicity
and heat capicity 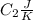 .
.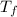 .
.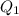 heat is extracted by the heat engine from the reservior
heat is extracted by the heat engine from the reservior 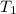 to
to 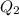 heat is rejected by the heat engine to the reservior
heat is rejected by the heat engine to the reservior 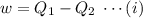
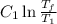 .
.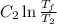 .
.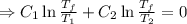
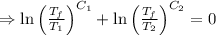
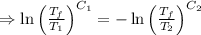
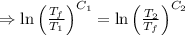
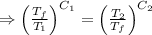 [taking anti-log both the sides]
[taking anti-log both the sides]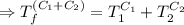
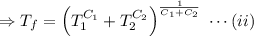
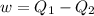
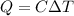
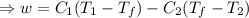
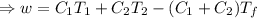
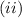 ,
,