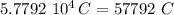Physics, 20.09.2020 17:01 garzamatt7
What is the total negative charge, in coulombs, of all the electrons in a small 1.20 g sphere of carbon? One mole of C is 12.0g, and each atom contains 6 protons and 6 electrons. Attempted to calculate the total number of electrons first and then using that to calculate the total negative charge. Came out with 8475.4, but MP says it is wrong.

Answers: 3
Another question on Physics

Physics, 22.06.2019 08:30
What properties of a moving object are used in determining the object's energy of motion
Answers: 2

Physics, 22.06.2019 13:30
If the spring constant k of a pogo stick is 3500 n m and the weight of the person on the pogo stick is 700 n, how much is the spring in the botom of the pogo stick compressed?
Answers: 2

Physics, 22.06.2019 14:10
Click the game tab at the bottom of the simulation and select level 1. (there is no seesaw balance for this part of the activity.) balance the first equation, and click check to see if you got it right. if you can’t balance it in the first try, you can try again. work through the five equations for level 1. click continue to go on to level 2, and later level 3. each level is more difficult than the one before. keep trying until all the equations are balanced. in one or two sentences, describe how you did in the balancing game. in a few more sentences, explain one strategy you learned for balancing more complex equations.
Answers: 2

Physics, 22.06.2019 18:00
Directions: count the number of atoms. ar co2 na3po4 so3 nac2h3o2
Answers: 1
You know the right answer?
What is the total negative charge, in coulombs, of all the electrons in a small 1.20 g sphere of car...
Questions




Mathematics, 25.04.2020 03:19





Mathematics, 25.04.2020 03:19

History, 25.04.2020 03:19


History, 25.04.2020 03:19


History, 25.04.2020 03:19

History, 25.04.2020 03:19

Mathematics, 25.04.2020 03:19

History, 25.04.2020 03:19

Mathematics, 25.04.2020 03:19

Computers and Technology, 25.04.2020 03:19

Mathematics, 25.04.2020 03:19

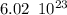 molecules
molecules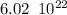 molecules.
molecules.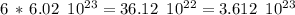 electrons.
electrons.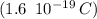 , and we obtained the total negative charge of the carbon sample:
, and we obtained the total negative charge of the carbon sample: