
Physics, 20.09.2020 15:01 whatistheinternetpas
You place an ice cube of mass 7.50×10−3kg and temperature 0.00∘C on top of a copper cube of mass 0.540 kg. All of the ice melts, and the final equilibrium temperature of the two substances is 0.00∘C. What was the initial temperature of the copper cube? Assume no heat is exchanged with the surroundings.

Answers: 3
Another question on Physics

Physics, 21.06.2019 18:50
How does the image distance (di) of a convex lens compare with the image distance of a concave lens? a. the image distance of the convex lens is positive, and that of the concave lens is negative. b. both are negative for a virtual image. c. both are positive for a virtual image d. the image distance of the convex lens is negative, and that of the concave lens is positive.
Answers: 1

Physics, 21.06.2019 22:50
If the temperature were raised very high, classically what would we expect the heat capacity per object to be for this one-dimensional system? give a numerical value. chigh t = __ j/k/object (one reason for the discrepancy is that the high-temperature limit assumes that the number of oscillators is large (n > > 1), which is not the case in this tiny system.)
Answers: 2

Physics, 22.06.2019 01:00
15. give an example for some particles or waves that are moving faster than light in everyday life 16. what is a laser? 17. what is an oscilloscope? 18. what does it means practically that nothing is faster than light in vacuum? 19. what is vacuum?
Answers: 2

Physics, 22.06.2019 04:00
Amodel rocket with a mass of 0.212 kg is launched into the air with an initial speed of 84 m/s. how much kinetic energy will the rocket have at a height of 214 m? assume there is no wind resistance. 634 j 303 j
Answers: 2
You know the right answer?
You place an ice cube of mass 7.50×10−3kg and temperature 0.00∘C on top of a copper cube of mass 0.5...
Questions


Mathematics, 10.03.2020 21:37

English, 10.03.2020 21:37

History, 10.03.2020 21:37






Mathematics, 10.03.2020 21:38








English, 10.03.2020 21:40


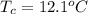
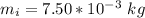
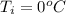
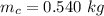
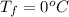
![Q = m_c * c_c * [T_c - T_f ]](/tpl/images/0771/9055/7a9ed.png)
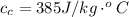
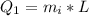
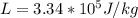
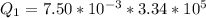
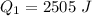
![2505 = 0.540 * 385 * [T_c - 0 ]](/tpl/images/0771/9055/f1873.png)


