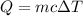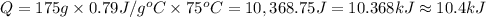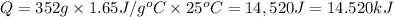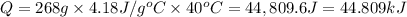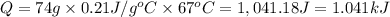Idon't really understand. worth 12 points!
heat = mass(m) * specific heat capacity(c) * temp....

Physics, 24.09.2019 08:20 chefjones06p0gvlh
Idon't really understand. worth 12 points!
heat = mass(m) * specific heat capacity(c) * temp. change(delta t)
itembank:
a.) 1.04kj
b.) 10.4 kj
c.) 14.5 kj
d.) 44.8 kj
match each item from the item bank to its corresponding match:
1.)heat = mass * c * temp change
175g 0.79j/gc 75 c
2.)heat = mass * c * temp change
352g 1.65j/gc 25 c
3.)heat = mass * c * temp change
268g 4.18j/gc 40 c
4.)heat = mass * c * temp change
74g 0.21j/gc 67 c
i don't have to have the answer to all of them; i just need understanding what to do.

Answers: 2
Another question on Physics

Physics, 22.06.2019 09:00
In a heat engine if 1000 j of heat enters the system the piston does 500 j of work, what is the final internal energy of the system if the initial energy was 2000 j? 1. write the equation 2.list out your known variables 3.plug the numbers into the equations 4.solve 5.write your solution statement that includes initial energy and final
Answers: 1


Physics, 22.06.2019 12:00
In an experiment, how can i change human errors? be specific.
Answers: 1

Physics, 22.06.2019 14:20
4r-134a enters the condenser of a residential heat pump at 800 kpa and 50°c at a rate of 0.022 kg/s and leaves at 750 kpa subcooled by 3°c. the refrigerant enters the compressor at 200 kpa superheated by 4°c determine (a) the isentropic efficiency of the compressor, (b) the rate of heat supplied to the heated room, and (c) the cop of the heat pump. also, determine (d) the cop and rate of heat supplied to the heated room if this heat pump operated on the ideal vapor-compression cycle between the pressure limits of 200 and 800 kpa. (0.757, 4.37 kw, 5.12, 6.18, 3.91 kw)
Answers: 3
You know the right answer?
Questions

English, 21.10.2020 21:01






History, 21.10.2020 21:01

Mathematics, 21.10.2020 21:01




Chemistry, 21.10.2020 21:01

Chemistry, 21.10.2020 21:01

Computers and Technology, 21.10.2020 21:01




Mathematics, 21.10.2020 21:01