
Physics, 08.10.2020 01:01 ashtyndowdle
2. The visible region of the hydrogen spectrum results from relaxation of electrons from excited states to energy level 2 (n1). Use the Rydberg equation and your measured wavelengths to determine the energy transitions associated with each of your observed wavelengths for hydrogen. In other words, calculate the excited state energy level (n2) for each of your observed wavelengths for hydrogen. n has integer values; so, calculate it first with appropriate significant digits, then round it to an integer. Use the key to show your work for at least one calculation. Must show energy levels for each hydrogen wavelength.

Answers: 2
Another question on Physics

Physics, 22.06.2019 16:00
The frequency of the fundamental of the guitar string is 320 hz. at what speed v do waves move along that string?
Answers: 2

Physics, 22.06.2019 19:40
12. a body is a particular amount of matter. it can be a solid, liquid or gas. it can be described as existing in a. size and shape. b. motion and force c. time and space. d. location and movement.
Answers: 2


Physics, 23.06.2019 00:50
Which of the following is not an appropriate unit for acceleration? (kg/12 mihr? m/min/sec cm/30c?
Answers: 2
You know the right answer?
2. The visible region of the hydrogen spectrum results from relaxation of electrons from excited sta...
Questions


Mathematics, 19.06.2020 23:57


Mathematics, 19.06.2020 23:57



Mathematics, 19.06.2020 23:57






Biology, 19.06.2020 23:57


Mathematics, 19.06.2020 23:57

Mathematics, 19.06.2020 23:57




Mathematics, 19.06.2020 23:57

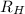 (1/2² - 1 / n²)
(1/2² - 1 / n²)



