
Physics, 15.10.2020 09:01 Onlyoneeniyaaa
A large research balloon containing 2.00 × 10^3 m^3 of helium gas at 1.00 atm and a temperature of 15.0°C rises rapidly from ground level to an altitude at which the atmospheric pressure is only 0.900 atm. Assume the helium behaves like an ideal gas and the balloon’s ascent is too rapid to permit much heat exchange with the surrounding air.
Required:
a. Calculate the volume of the gas at the higher altitude.
b. Calculate the temperature of the gas at the higher altitude.
c. What is the change in internal energy of the helium as the balloon rises to the higher altitude?

Answers: 3
Another question on Physics

Physics, 21.06.2019 19:30
Contrast the force of gravity between these pairs of objects a 1 kg mass and a 2 kg mass that are 1 m apart and two 2 kg masses that are 1 m apart
Answers: 2

Physics, 21.06.2019 20:00
Alice added sodium chloride to water and stirred the water for several minutes. alice is most likely trying to demonstrate that ionic compounds a. are hard. b. can dissolve. c. are clear. d. can melt.
Answers: 1

Physics, 22.06.2019 04:30
The current in a hair dryer measures 11 amps. the resistance of the hair dryer is 12 ohms. what is the voltage?unit:
Answers: 1

Physics, 22.06.2019 20:30
Ahockey player of mass 82 kg is traveling north with a velocity of 4.1 meters per second he collides with the 76 kg player traveling east at 3.4 meters per second if the two players locked together momentarily in what direction will they be going immediately after the collision how fast will they be moving
Answers: 2
You know the right answer?
A large research balloon containing 2.00 × 10^3 m^3 of helium gas at 1.00 atm and a temperature of 1...
Questions


World Languages, 10.10.2021 14:00



History, 10.10.2021 14:00



Health, 10.10.2021 14:00




English, 10.10.2021 14:00

English, 10.10.2021 14:00

Chemistry, 10.10.2021 14:00

Business, 10.10.2021 14:00

Chemistry, 10.10.2021 14:00


English, 10.10.2021 14:00

Mathematics, 10.10.2021 14:00

Chemistry, 10.10.2021 14:00

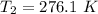
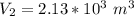
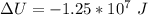
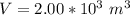
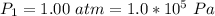
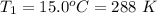
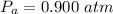
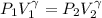
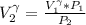
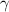 is a constant with value
is a constant with value 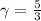 for an ideal gas
for an ideal gas 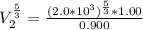
![V_2 = (\sqrt[5]{103.14641852} )^3](/tpl/images/0807/0306/7603b.png)
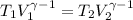
![T_2 = 288 * [\frac{2 * 10^{3}}{ 2.13 *10^{3}} ]^{ \frac{5}{3} -1 }](/tpl/images/0807/0306/ce7bb.png)
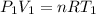
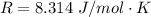
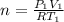

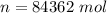
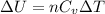
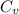 is the specific heats of gas at constant volume and the value is
is the specific heats of gas at constant volume and the value is 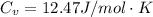
![\Delta U = 84362 * 12.47 * [T_2 - T_1 ]](/tpl/images/0807/0306/1f924.png)
![\Delta U = 84362 * 12.47 * [276.1 - 288 ]](/tpl/images/0807/0306/0b18f.png)


