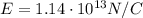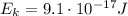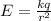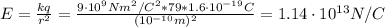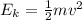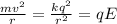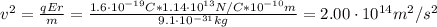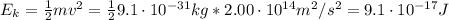Physics, 19.10.2020 20:01 Carrchris021
Before the development of quantum theory, Ernest Rutherford's experiments with gold atoms led him to propose the so-called Rutherford Model of atomic structure. The basic idea is that the nucleus of the atom is a very dense concentration of positive charge, and that negatively charged electrons orbit the nucleus in much the same manner as planets orbit a star. His experiments appeared to show that the average radius of an electron orbit around the gold nucleus must be about 10−1010−10 m. Stable gold has 79 protons and 118 neutrons in its nucleus.
What is the strength of the nucleus' electric field at the orbital radius of the electrons?
What is the kinetic energy of an electron in a circular orbit around the gold nucleus?

Answers: 1
Another question on Physics

Physics, 22.06.2019 12:10
The average density of the planet uranus is 1.27 103 kg/m3. the ratio of the mass of neptune to that of uranus is 1.19. the ratio of the radius of neptune to that of uranus is 0.969. find the average density of neptune.
Answers: 1

Physics, 22.06.2019 15:50
Ryan is examining the energy of the particles in a bar of gold. what is ryan most likely studying?
Answers: 1

Physics, 22.06.2019 22:10
7. see worksheet 1 for values of variables x1, x2 and x3 and answer the following questions: a. for each variable find the mean, median, coefficient of skewness, range and population standard deviation. b. compared to variable x1, how are the mean and median affected by extreme values (outliers) seen in x2 and x3. c. is the median or mean the better measure of location for x2 and x3? explain. d. explain the differences in the magnitudes of the skewness coefficients for the three variables. e. what is the relationship between the range and standard deviation looking across the three variables?
Answers: 1

Physics, 23.06.2019 01:00
The amount of heat required to change liquid water to vapor at its boiling temperature is 2256 kj/kg. the amount of heat required to change liquid mercury to its vapor state at its boiling temperature is 295 kj/kg. one kg of each substance is currently at its boiling point. how will the amount of thermal energy required to change each substance from a liquid to a gas differ?
Answers: 3
You know the right answer?
Before the development of quantum theory, Ernest Rutherford's experiments with gold atoms led him to...
Questions